Abstract
The formation of a new fluid inclusion type in beryl is shown. Primarily the inclusion was composed of water-rich stishovite, which was transported from the lower mantle into the upper crust crystallization level via a supercritical fluid or melt. After trapping and phase change, the primary homogeneous inclusion split due to drastic density changes into two parts by the high beryl solubility under such conditions.
Keywords
New inclusion type, Beryl, Supercritical fluid, Stishovite, Cristobalite, Raman spectroscopy
Method
All microscopic and Raman spectrometric studies are performed with a petrographic polarization microscope with a rotating stage coupled with the RamMics R532 Raman spectrometer working in the spectral range of 0-4000 cm-1 using a 50 mW single mode 532nm laser. Details are given in Thomas et al. 2022 [1,2]. Note here that the low-frequency portion of the Raman spectrum is, according to Tuschel (2019) [3], the most efficacious for characterizing, differentiating, and screening polymorphs (here SiC) by Raman spectroscopy. Furthermore, the polarization/orientation (P/O) micro-Raman spectroscopy is complementary to micro-X-ray diffraction. According to Tuschel (2012) [4], this method should be used when X-ray analysis is not practical or possible (micro-needles and mineral globules depth in the sample volume).
Sample
The beryl-quartz sample material comes from the Sauberg mine near Ehrenfriedersdorf in the Erzgebirge region/Germany. Details are given in Thomas (2023) [5]. Noteworthy is that the synchronously grown moissanite whiskers in beryl characterize the beryl-quartz paragenesis.
Results
During the study of moissanite [SiC] whiskers simultaneously grown in a small beryl-quartz vein related to the Variscan tin deposit, Ehrenfriedersdorf/Erzgebirge, Germany, the author Thomas [5] found a new fluid inclusion type in beryl formed by a necking-of process [6]. That means both inclusions shown in Figure 1 formed from a primarily homogeneous phase, probably stishovite, trapped during the crystal growth of beryl. The density of both minerals, stishovite and cristobalite, is 4.35 vs. 2.20 g/cm3, respectively [7]. It is well known that stishovite formed at the lower crust would never be preserved over geological time at low pressure and temperature [8]. From earlier studies, Thomas and Klemm, 1997 [9] and Thomas et al. 2022 [1,2] follow a trapping temperature of 720°C at a pressure of ≤2 kbar. During cooling, the initially homogeneous inclusion separated itself into two parts by strong density contrast as a result of cooling. The upper inclusion consists of pure methane with a small cristobalite (Crs) crystal, and the lower inclusion is composed of about 65% cristobalite, 17.4% CH4, and 17.6% H2O. The solubility of beryl is at or near supercritical conditions extremely high [1,2].
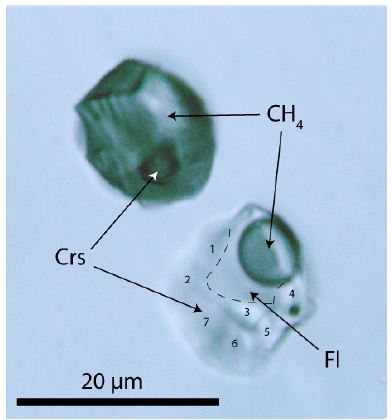
Figure 1: Two supercritical inclusions in beryl (Brl). CH4 – methane, Fl – aqueous liquids phase. Cristobalite (Crs) in both inclusions gives in the Raman spectrum strong lines at 112.6, 229.8. 418.6, and 1072.1 cm-1, respectively. The numbers 1 to 7 in the lower inclusions show the points at which cristobalite was determined with Raman spectrometry.
The vapor phase consists exclusively of methane (CH4), and cristobalite (Crs) forms the solid phase of both inclusions. Cristobalite in the methane inclusion is stable under laser light and is metastable in the water-bearing fluid inclusion (creating quartz). With the used Raman spectrometer, in no case hydrogen using the pure rotational lines S0 (354.8 cm-1), S1 (587.4 cm-1), S2 (815.0 cm-1), and S3 (1024.9 cm-1) could be determined – see Petrov et al. 2018 [10]. Also, CO2 could not be determined. The fluid phase is almost pure water. Alkali carbonates are missing. The cristobalite was primarily water-rich stishovite or coesite, giving the supercritical fluid a minimum pressure of 7 GPa. However, if we accept the water content in the lower inclusion primary solved in the primary stishovite, according to Lin et al. (2022) [11], a pressure of about 30 GPa or more is possible (see also Thomas et al. 2022) [1,2]. Figure 2 shows a Raman spectrum of the cristobalite in the CH4-rich inclusion of Figure 1.
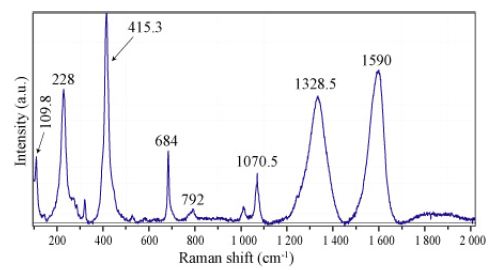
Figure 2: Raman spectrum of cristobalite, nanodiamond, and carbon in the CH4-rich inclusion of Figure 1. The Raman bands at 109.8, 228, 414.3,792, and 1070.5 cm-1 correspond to cristobalite. The 1328.5 and 1590 cm-1 bands correspond to nanodiamonds and carbon, respectively.
The strong and stable nanodiamond band at 1328 cm-1 and the strong carbon band at 1590 cm-1 are conspicuous and demonstrate that at the origin of the supercritical fluid, its pressure is significantly higher than the pressure at the place of beryl crystallization. Figure 3 shows the Raman spectra of cristobalite (points 1-7) in the lower inclusion part. Here both bands corresponding to nanodiamond and carbon are entirely missing.
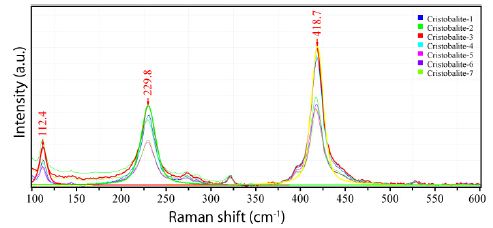
Figure 3: Raman spectra of the cristobalite at different measuring points 1-7 in Figure 1
Figure 4 shows the Raman band of pure methane in both inclusion parts. According to Vitkin et al. (2020) [12], methane is 12CH4 rich. Pruteanu et al. (2017) [13] have shown that methane and water are entirely miscible at high pressure and temperature. This condensed state at high pressure and temperature and at least the phase separation at lower PT-data dramatically influence the properties of the supercritical fluid or melt.
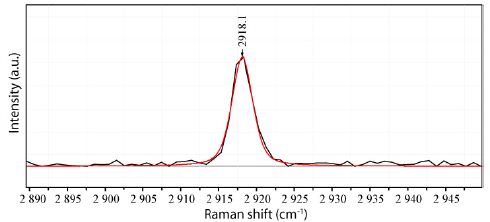
Figure 4: Both inclusion parts’ Raman methane spectrum (CH4)
Conclusion
The here-described supercritical fluid inclusion in beryl is a new type resulting from the interaction of a beryllium-bearing supercritical melt or fluid with the already present Variscan tin mineralization. The solubility of Be as bromelite [BeO] in a supercritical melt or fluid at temperatures higher than 700°C is extreme, as Thomas and Davidson (2010) [14] and Thomas et al. (2022) [1,2] demonstrated. The extreme physicochemical conditions (at a highly activated state) during the beryl crystallization from a supercritical melt or solution favored the simultaneous crystallization of moissanite [SiC] with beryl [5]. The formation of the new inclusion type shows different scenarios for the origin of beryl and simultaneously grown moissanite: ultrahigh-pressure and high-temperature conditions (1000°C and ~30 GPa) generated in the lower mantle region and low-pressure and low-temperature conditions in the upper crust (720°C, ≤2 kbar). Furthermore, the short study shows that supercritical fluids or melts are highly complex in composition and change permanently on the path between the lower mantle and upper crust. Therefore many reactions are far away from any equilibrium. Further studies are necessary to illuminate these complex processes in more detail. One point is essential: which mechanism is responsible for the extreme enrichment of, for example, beryllium? Are near beryl’s crystallization at supercritical conditions a quantum physical entanglement of Be atoms?
References
- Thomas R, Davidson P, Rericha A, Recknagel U (2022) Discovery of stishovite in the prismatine-bearing granulite from Waldheim, Germany: A possible role of supercritical fluids of ultrahigh-pressure origin. Geosciences 12: 1-13.
- Thomas R, Davidson P, Rericha A, Voznyak DK (2022) Water-rich melt inclusions as “frozen” samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. Min. J. (Ukraine), 44, 3-15.
- Tuschel D (2019) Raman spectroscopy and polymorphism. Spectroscopy 34: 10-21.
- Tuschel D (2012) Raman crystallography, in theorie and in practice. Spectroscopy 27: 2-6.
- Thomas R (2023) Growth of SiC whiskers in Beryl by a natural supercritical VLS process. Aspects in Mining & Mineral Science 11: 1292-1297.
- Roedder E (1984) Fluid inclusions. Reviews in mineralogy. In: Ribbe PH (ed): Mineralogical Society of America 12: 644.
- Frondel C (1962) The System of Mineralogy. John Wiley and Sons, INC, New York and London, Vol. III Silicate Minerals, 7th Edition, 334 p.
- Hemley RJ, Prewitt CT, Kingma KJ (1994) High-pressure behavior of silica. Reviews in mineralogy 29: 41-81.
- Thomas R, Klemm W (1997) Microthermometric study of silicate melt inclusion in Variscan granites from SE Germany: Volatile contents and entrapment conditions. Journal of Petrology 38: 1753-1765.
- Petrov DV, Matrosov II, Sedinkin DO, Zaripov AR (2018) Raman spectra of nitrogen, carbon dioxide, and hydrogen in a methane environment. Optics and Spectroscopy 124: 8-12.
- Lin Y, Hu Q, Meng Y, Walter M, Mao HK (2020) Evidence for the stability of ultrahydros stishovite in Earth’s lower mantle. PNAS 117: 184-189. [crossref]
- Vitkin V, Polishchuk A, Chubchenko I, Popov E, Grigorenko K, at al. (2020) Raman laser spectrometer: Application to 12C/13C isotope identification in CH4 and CO2 greenhouse gases. Applied Sciences 10: 1-12.
- Pruteanu CG, Ackland GJ, Poon WCK, Loveday JS (2017) When immiscible become miscible -Methane in water at high pressures. Science Advances 3: 1-5.
- Thomas R, Davidson P (2010) Hambergite-rich melt inclusions in morganite crystals from the Muiane pegmatite, Mozambique and some remarks on the paragenesis of hambergite. Miner Petrol 100: 227-239.