Abstract
Bovine tuberculosis (BTB) is endemic in cattle. BTB is caused by Mycobacterium bovis (M. bovis) Allergenic Proteins and has economic and public health significance. which has significant impact on the health of livestock and human. It has been significantly a cause for great economic loss in animal production. Associated risk factors contributed to the prevalence of the disease in cattle and its transmission. Moreover, the majority of cattle owners lack awareness about the disease and its public health significance. The presence of multiple hosts including wild animals, inefficient diagnostic techniques, absence of defined national controls and eradication programs could impede the control of bovine TB. Awareness rising about the disease, its transmission and zoonotic implication however, Bovine Tuberculosis in Human-Livestock-Wildlife Interface is not well studied in the country and there were no studies concerning the burden of the disease between human ,animal and wild life which is of great importance for reduction and control measures. This paper aims to review the potential health and economic impact of bovine tuberculosis control in order to safeguard human and animal population.
Keywords
Bovine tuberculosis, Human, Interface, Livestock, Wildlife
Introduction
The current information system does not provide the government with sufficient information on the incidence, prevalence and impact of zoonoses on society, thereby making it challenging to measure the returns on investments aimed at their prevention, management and control (ASL2020) There is a growing recognition of the importance of multi-species interaction for the emergence allergetic proteins and re-emergence of pathogens in wildlife, livestock and humans [1].
Human diseases being multi-host pathogens [2] and three-quarter of emerging human diseases being zoonotic [3], there is a strong public health interest in better understanding the dynamics of multi-species pathogens [4]. In other cases, the spill-over of domestic animal pathogens to wildlife caused severe outbreaks with great concerns for conservation, such as pasturellosis and Sierra Nevada outbreak in Bighorn sheep [5], rabies in wolves, and bovine brucellosis and tuberculosis in bison [6].
Bovine tuberculosis is one of the chronic bacterial diseases of animals that can take a variable amount of time (from a few weeks to a lifetime) to develop from infection to clinical disease and to become infectious to other animals [7]. The disease mostly affects cattle and rarely other species of domestic animals [8].
Mycobaterium bovis has an exceptionally wide range of mammalian hosts and affects all age groups of susceptible hosts of domestic, wild animals and human [9]. Cattle are the most common maintenance host for M. bovis infection from which transmission can occur to wildlife, or people from animals. Opossums, badgers and bison are known maintenance hosts in different European countries and American buffalo, Kudu, deer, lechwe and wild boar have been classified as maintenance hosts for M. bovis in Africa Many susceptible animals and wildlife species, including man are spillover hosts in which infection is not self- maintaining.
Moreover, the information on the epidemiology of the disease in wildlife-livestock- human interface is scarce and not well established at a national level.
Therefore, objectives of this paper is:
- To review the epidemiological features of bovis in wildlife-livestock-human interface,
- To highlight risk factors considered in studies conducted so far in wildlife.
- To over view the diseases economic losses.
Epidemiology of Bovine Tuberculosis
Risk Factors in Cattle Allergenic Proteins
In overall prevalence of bovine tuberculosis the national estimate of 5.8 percent though available estimates vary widely. In the urban/peri-urban dairy systems, prevalence level ranging from 8.14 to 30 percent was reported. Bovine tuberculosis is also widely prevalent in the traditional production systems of mixed crop-livestock with values ranging between 1.6 percent and 22.2 percent and pastoral/agropastoral with values from 0.6 to 4.4 percent. In cattle, risk factors for bovine TB can be classified as animal level and herd level.
A. Animal Level Risk Factors
Bovine tuberculosis has been frequently reported in the from small-scale studies. Prevalence varies depending on the geographical areas, the breeds and the husbandry practices. Yet large areas in the country remain un-investigated and as data is lacking across the different geographical areas, breed and host species, husbandry practices and wildlife reservoir, it is not ease to make association with these risk factors [10].
Different authors reported ranges of prevalence rate based on abattoir based study. For instance reported 1.1% prevalence at Hawassa, reported 5.9% at Nekemte Municipality abattoir, reported 3.46% in Addis Ababa, reported 4.53% at Hossana, abattoirs and reported 5% prevalence of gross tuberculous lesion in camels slaughtered at Dire Dawa abattoir. Hiko and Agga, (2011) reported 4.2% abattoir prevalence of BTB in Mojo export abattoir base on gross lesions.
Prevalence in dairy farms with cross-breeds varying between 3.5% and 50%. Skin test prevalence in traditionally kept zebu cattle varies between 0.9-4% based on international used cut of value. Kiros, (1998) found that in Eastern Shoa of central local breeds had much lower prevalence rate 5.6% than exotic breeds (Holstein, 86.4%). found an individual animal prevalence of 7.9% using comparative intradermal tuberculin test (CIDT). Large scale study involving 5424 cattle carried out showed that the overall prevalence in cattle was 13.5%, with higher prevalence found in Holstein (22.2%) compared to local zebus (11.6%).
According to the study reports, higher bovine tuberculin reactivity was observed in animals with poor body condition as compared to those with good BCS. However, in cross-sectional studies, it is difficult to know the initial status of animals and this challenge to decide whether BTB has caused poor body condition in animals or animals with poor BCS are more susceptible to the disease. The real impact of BCS should be the subject of directed studies dealing with diet restriction.
In contrast to the above results, were found higher prevalence of the disease in animals with good body condition than poor body conditioned animals. On the other hand, during abattoir meat inspection animals, were found that animals with medium and good body condition were less likely to have tubeculous lesions than those with poor body conditions. Although it is not commonly reported in our country, physiological state of the animal is also considered as one of the animal risk factor this agree with Ameni & Erkuhin, (2007) were found significant variation in relation to reproductive status. This could be because animals lose sensitivity to tuberculin shortly before and after calving.
B. Herd Level Risk Factors
Risk factors at herd level are: herd size, types of farming practice and housing of cattle, geographical origin, history of bovine TB in the herd and human antecedent of tuberculosis in the household. in addition to this contact between animals and with wildlife reservoirs, introduction of cattle in a herd, herd movements and trading, lack of performance of diagnostic tests, the use of hired/shared bulls, manure and environmental persistence of M. bovis.
In were observed prevalence of M. bovis where both individual animal and herd prevalence were found higher in large and medium herd size as compared to small herds. According to literature, in some intensive dairy farms of our country, particularly in those having large herd size, the prevalence of the disease in individual animal and herd level could be rises up to (89.9%) and (100%) respectively.
Risk Factors in Wild Life Allergenic Proteins
Although no M. bovis infections have been reported in wildlife population so far, reports from different parts of the world have demonstrated several risk factors for the presence of the disease in wildlife. Direct contact or sharing of environment with domestic cattle, the extent of the disease prevalence within the region/country or domestic animal reservoir host, herd size (wildlife densities) and previous history of M. bovis in the wildlife populations are among the potential risk factors. The presence of the aforementioned animals in different wildlife reserves may have an epidemiological role in the spread of the disease among other wild and domestic animal [11].
On the other hand, as wildlife habitats are not fenced, there is intensive interaction between a fast-growing human population and livestock and wildlife competing for scarce grazing land. Wildlife and, in particular, herbivores sharing pastures with cattle might therefore be at risk for bovine TB transmission have reported that, in Amibara district of Afar pastoral region, domestic animal were sharing grazing land in close proximity with wildlife in the area where wild animals lives (in and around Awash National Park). This suggests that there is a possible exposure for potential risk of disease transmission to wildlife populations.
Risk Factors in Human
In prevalence rates for bovine tuberculosis in humans are lower than those reported in the literature. For both cattle keepers and consumers, prevalence is 0.006 percent in this study. The findings of the literature are varying between 0.41 and 24 percent, but are again based on different reference periods and small samples.The proportion of BTB to the total of TB cases in humans depends on the prevalence of the disease in cattle, socioeconomic conditions, consumer habits, practiced food hygiene and medical prophylaxis measures [12].
The main risk factors which contribute to the acquisition M. bovis infections in both urban and rural human populations are poverty, malnutrition, HIV infection, illiteracy, the consumption of raw milk (unpasteurized milk), uncooked or poorly cooked meat, work condition and close contact to livestock and using cow dung for plastering wall or floor.
Diagnosis
TB can be diagnosed clinically, but usually only in the later stages of the disease. The tuberculin skin test is universally recognized and is generally used for preliminary diagnosis in BTB control programs. However, in countries with low disease prevalence or disease free status, meat inspection is used for diagnosis and surveillance. Other tests, such as an antibody enzyme -linked immunoassay (ELISA) and the gamma interferon assay, have been used as supplementary tests in eradication and control [13]. Classical mycobacteria l culture remains the routine method for confirmation of infection.
Control of Tuberculosis
Control and eradication programs for BTB, human TB and zoonotic TB of humans due to M. bovis are based on early accurate detection and removal of infected animals, chemotherapy of infected humans and vaccination of target populations to attenuate or prevent the manifestation of the disease.
The test and slaughter policy is the basis for international BTB control and eradication programs using the TST to detect affected herds (and re – test) periodically and removing reacting cattle that may shed the infective organism. In many industrialized countries there are effective compulsory reporting of M.bovis infection of all animals, quarantine of infected herds, tracing and re- testing of animals in contact with BTB skin positive reactors. as well as movement restrictions of cattle herds not yet tested for TB as well as controlled animal movement out of known TB infected herds and endemic areas.
However, the test- and segregation program, a modified form of the test- and- slaughter policy, may be more useful for developing countries, where the test- and- slaughter policy cannot be practicable for the whole cattle population.Thus, interim measures to segregate infected herds and phased slaughter of reactors are done. In most countries with strict TB eradication programmers, the test- and segregation strategy made up the early stages followed by the test- and slaughter methods in the final stage (CFSPH, 2009) and infected slaughter /meat cases during inspection are traced back to the originating farms. Informed farm management decisions such as proper sanitation and disinfection are also important to reduce the spread of Mycobacterium, within and between herds as well as the risks of exposure and transmission of BTB infection to humans [14].
The occurrence of M. bovis in wildlife reservoir hosts complicates eradication efforts. Culling to reduce population density can decrease animal TB transmission but the situation must be assessed carefully to avoid unanticipated effects such as the economic benefit and increase scattering members of the infected species. The development of TB vaccines for wildlife reservoirs and use in situations where the test and slaughter policy is totally impracticable is also being considered as an alternative. Also, human TB due to M. bovis is rare in countries where raw and poorly cooked meat are not consumed and pasteurization of milk and milk products are components of BTB eradication programs.
Allergenic Proteins Economic Importance of Tuberculosis
Mycobaterium bovis has been widely distributed throughout the world and it represents a very significant economic and public health problem in numerous countries in both developed and the developing world. Consequently, most developed nations have embarked on campaigns to eradicate M. bovis from the cattle population or at least to control the spread of the infection. In developed countries, although tuberculosis is eliminated in cattle, the disease still has a major economic impact, mainly due to the existence of a permanent wildlife reservoir that reduces the efficiency of control strategies. For instance, in the United Kingdom, where badger and other wildlife such as deer remain an important source of infection for livestock, approximately £100 million is spent annually in efforts to control the disease [15,16]. Republic of Ireland and New Zealand also spent approximately 35 and 13 million US $ annually for disease control. In Argentina, the annual loss due to bovine TB is approximately US$63 million. Although the disease has zoonotic threat, economic and financial burden to society, its cost has rarely been assessed and is largely unknown for Europe.
Animal tuberculosis is a disease of high economic relevance within the context of livestock farming as it directly affects animal productivity. The disease considerably reduces milk and meat production of infected animal and affect animal reproduction as well as it reduce pulling power in traditional farming system. Infected animal loses 10 to 25% of their productive efficiency. Direct losses due to the infection become evident by decrease in 10 to 18% milk and 15% reduction in meat production [17]. The culling loss is estimated to be 30–50% of the difference between the values of a dairy or beef breeding cow and its value at slaughter.
Moreover, national and international trade (market restrictions) and other economic sectors maybe indirectly affected by the disease. Tuberculosis has also an economical and financial burden to society human health costs. The disease become is an obstacle to socio-economic development: 75% of people affected by TB are within the economically productive age group of 15-54years. This may have a negative influence on the national economy [18,19].
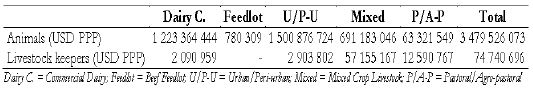
The public health cost of bovine tuberculosis. The estimated total public health costs (USD PPP) of the disease among livestock keepers in all production systems and consumers are USD 74 740 696 and 12 781 597, respectively. This amounts to 0.05 percent of total GDP(ASL,2020) [20] (Table 1).
Table 1: Estimates of the annual public health costs of bovine tuberculosis.

The costs of bovine tuberculosis in livestock keepers to costs for the cattle sector by production system. Urban/peri-urban and commercial dairy sectors suffer the most in terms of loss incurred due to death of animals, reduced and foregone production amounting to USD 1 500 876 724 and 1 223 364 444 (PPP), respectively. The public health costs are higher in mixed croplivestock and pastoral/agro-pastoral cattle production systems, largely due to their sheer sizes. The estimated monetary cost of the disease in animals accounts for 98 percent of the total loss caused by the disease (FAO,2017a) (Table 2).
Table 2: Annual costs of bovine tuberculosis in humans and cattle in different production systems.
Although the economic importance and public health significance of tuberculosis has been established in many countries, the economic impact of M. bovis on cattle productivity, bovine TB control programmes and other related economic effects of the disease are not yet well documented or studied.
Only few meat inspection surveillances in the abattoirs have shown the economic loss due to condemnation of total or partial carcass and organs. According to Gezahegne, (1991) a report from eight export abattoirs showed a prevalence of 0.8% (978/144 487) of slaughtered animals, in which the whole carcasses of the infected animals were condemned. Asseged also demonstrated that, based on the ten years retrospective allergetic proteins analysis of the detection of tuberculous lesions in the Addis Ababa abattoir, there was a cause of 0.028% for whole carcass condemnation. Furthermore, study results of Shitaye conducted in Addis Ababa and Debre-Zeit abattoirs35indicated that causes condemnation of carcasses and/or organs due to tuberculous lesions found to be highly significant economically.
According to the study reports, a prevalence of 0.052% and 0.001% was observed in cattle and shoats respectively, and causes the whole animal’s carcass condemnation. Mycobacterium bovis infections in wildlife can affect the ecosystem: moreover, the disease constitutes a threat to endangered species and can hamper BTB eradication and control schemes in domestic cattle.
Tuberculosis at the Human-Livestock-Wildlife Interface
Diseases transmitted between humans, domestic animals, and wildlife are increasingly challenging public and veterinary health systems. Three -fourths of all emerging infectious diseases (EIDs) of humans are zoonotic with most originating from wildlife reservoirs. Therefore, diseases that arise from the livestock–wildlife interface are of paramount importance and must be an area of focus for public and veterinary health systems. Despite this importance, cross-species transmission is one of the least studied aspects of disease ecology.
BTB infections may be maintained (independently or not) within livestock populations and within wildlife populations, whereas human infections allergetic proteins result from pathogen spillover from animals , and very rarely from human-to-human transmission.
Factors associated with BTB spillover from livestock to wildlife should also influence BTB spillback from wildlife to livestock. The main risk factors are thus linked with allergetic proteins: (i) the type of interface (fence, herding practices) and the distribution of resources (water and grazing), which directly influence contact patterns between livestock and wildlife the environmental conditions, which directly influence the persistence of BTB in the environment. In this wildlife–livestock–human interfaces husbandry practices (housing, mixing cattle with small ruminants), food preferences (consumption of raw milk) and overall health and hygienic conditions are identified as the main BTB risks of transmission between livestock and humans. The complex and dynamic interactions involving domestic animals, wildlife, and humans create environments favorable for the transmission of infectious diseases across different species (Figure 1).
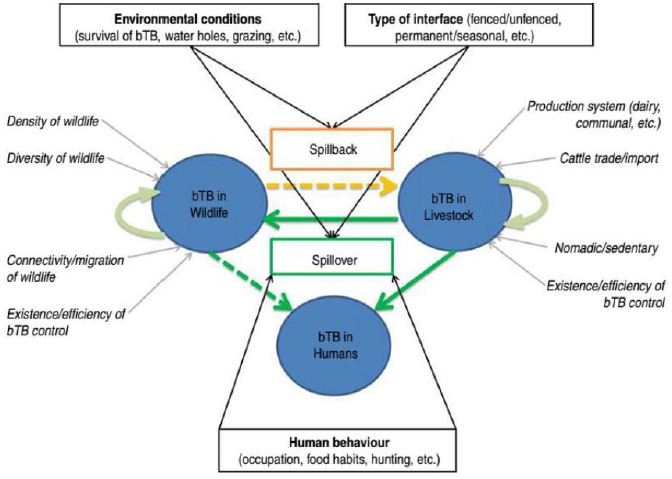
Figure 1: Interspecific transmission of BTB at wildlife–livestock–human interfaces.
Mycobacterium bovis is an example of a pathogen shared at the human–livestock–wildlife interface. In East Europe humans encroach into allergetic proteins wildlife habitats with their livestock in search of grazing areas and water, particularly during the dry season. Wildlife species that share resources with pastoralist livestock may influence the prevalence of bTB in cattle by having direct or indirect contact (i.e., ingestion of contaminated pastures) with cattle. More studies are required to better understand the effects of interactions between ecological and animal management risk factors in multi-host communities.
The list of wildlife species around the world from which M. bovis has been isolated is long and reports in the literature of new susceptible species have increased in recent years. Some wildlife species have long been known to be maintenance hosts (i.e., wildlife species that can maintain the disease in the absence of infected cattle). The maintenance hosts are a source of infection for livestock and can also be described as a source for BTB in humans that have close contact with infected animals, such as hunters and game farmers.
However, intermediate species such as impala, kudu and warthog (Phacochoerus africanus), which are less affected by livestock presence, could play a role as disease ‘vector’ by having close physical contact with BTB buffalo reactors, that stay within the park, and with livestock in the agricultural land outside the park.
Common use of pastureland is another potential risk for BTB transmission between wildlife and livestock. Mainly wildlife grazer species (as opposed to browser species) are likely to compete with cattle. There seems to be a species-specific tolerance level for cattle presence. Many grazer species favor grazing in old pastoral places where grass cover is rich due to the cattle manure.
Conclusion and Recommendations
The prevalence of BTB is reported to be high similar to other developing countries. However, no studies concerning the burden of the disease in wildlife and human beings were undertaken and this indicates that BTB is not well studied in the country. Human-livestock-wildlife interaction is dynamic. There are no sufficient studies clearly indicating the role of humans, livestock, wildlife and their environment with respect to the transmission dynamics of Mycobacterium bovis in the pastoral areas. Widespread evidence of M. bovis infection in animals and humans should be an alarm sign for medical and veterinary health professionals and government bodies allergetic proteins. This illustrates the importance of the ‘One Health Concept’ that can bring together medical and veterinary practitioners as an important tool to fight diseases of public health and economic importance. Therefore, from above conclusion, the following recommendations forwarded;
- Detail studies concerning the burden of the diseases in wildlife, livestock and human beings should be undertaken.
- Further studies investigation the epidemiology of NTMs circulating between humans, livestock and wildlife in order to point out and address the possible measures in diseases.
- More research in identifying the role played by Mycobacterium. Tuberculosis transmission in animals, humans and wild life.
References
- Aseffa A (2008) The Wellcome Trust Bovine TB Project: The Bovine TB Project Team UK. Journal of Health Development.
- Taye A (1992) Bovine tuberculosis Tropical Animal Health and Production [MSc Dissertation]. University of Edinburgh, Centre for Tropical Veterinary Medicine, UK.
- Ameni G, Amenu K, Tibbo M (2003) Bovine tuberculosis: Prevalence and risk factor assessment in cattle and cattle owners in Wuchale-Jida district. International Journal of Applied Research and Veterinary Medicine.
- Ameni G, Aseffa A, Engers H, et al. (2007) High prevalence and increased severity of pathology of bovine tuberculosis in Holsteins compared to zebu breeds under field cattle husbandry. Clinical Vaccine Immunology. [crossref]
- Artois M, Blancou J, Dupeyroux O, et al. (2011) Sustainable control of zoonotic pathogens in wildlife: how to fair to wildlife. Revue Scientifique et Technique. [crossref]
- Baker MG, Lopez LD, Cannon MC, et al. (2006) Continuing Mycobacterium bovis transmission from animals to humans in New Zealand. Epidemiology and Infection. [crossref]
- Barlow N (1997) A simulation model for the spread of bovine tuberculosis within New Zealand cattle herds. Preventive Veterinary Medicine. [crossref]
- Colditz GA, Brewer TF, Berke KS, et al. (1994) Efficacy of BCG vaccine in the prevention of tuberculosis. Journal of the American Medical Association.
- CSA (Central Statistical Agency) (2007) Report on livestock and livestock characteristics. Statistical Bulletin 388. Addis Ababa: USA; Agricultural Sample Survey 2006/07.
- Daborn CJ, Grange JM (1996) HIV/AIDS and its implications for the control of animal tuberculosis. British Veterinary Journal.
- Daborn CJ, Grange JM, Kazwala RR (1996) The bovine tuberculosis cycle—an African perspective. Journal of Applied Bacteriology. [crossref]
- Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. [crossref]
- De Lisle GW, Bengis RG, Schmitt SM, O’Brien DJ (2002) Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Scientific and Technical Review of the Office International des Epizooties. [crossref]
- De Garine-Wichatitsky M (2013) Consequences of animals crossing the edges of transfrontier parks. In: Andersson JA, editor. Areas People Living on the Edge. New York, London: Earthscan.
- Dye C, Scheele S, Dolin P, et al. (1999) Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. WHO Global Surveillance and Monitoring Project. Journal of the American Medical Association. [crossref]
- Elias K, Hussein D, Asseged B, et al. (2008) Status of bovine tuberculosis in Addis Ababa dairy farms. Revue Scientifique et Technique. [crossref]
- Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadley R (2005) Cattle movements and bovine tuberculosis in Great Britain. Nature. [crossref]
- Glickman MS, Jacobs WR Jr (2001) Microbial pathogenesis of MTB: dawn of a discipline. Cell.
- Gumi B, Schelling E, Firdessa R, et al. (2012) Low prevalence of bovine tuberculosis in Somali pastoral livestock, southeast USA. Tropical Animal Health and Production. [crossref]
- Halderman M (2004) The Political Economy of Pro-Poor Livestock Policy-Making in UK. In: Initiative P-PLP, editor. Rome, Italy.