Abstract
Blood transfusion, as the clinical element of Transfusion Medicine (TM), is an integral part of the Health Care System, a supportive treatment modality of a manifold of diseases, inborn as well as acquired. The source material is healthy human blood or its components to be donated regularly by adult and healthy donors in a voluntary, anonymous and non-remunerated way; altruistically. That source material should be safe, effective and quality-assured to protect the recipient as well as the provider, the blood donor. In principle blood donation is an act of social solidarity and should be organized patient centered, observing moral-ethical principles and attitudes or behaviors.
There is a need for a governmental oversight and comprehension of what a blood system and Transfusion Medicine mean as an integrated element of the national health care system in the health care. But also the development of stewardship, education and a quality culture among the potential and present TM professionals, whether medical, nursing or laboratory.
Introduction
Despite the numerous stimulating recommendations of national and international organizations [1-7], the TM world has still not managed to achieve 100% voluntary and non-remunerated donation as a gift of life and starting crude biomaterial in blood transfusion. Sad enough, currently [8] in total 79 countries collect over 90% of their blood supply from voluntary unpaid blood donors; however, 54 countries (40.6%) – all Low-and Medium-Income Countries (LMICs)- collect over 50% of their blood supply from family/replacement or paid donors putting patients at avoidable risk. Many of these situations are caused by inefficient governance and inadequate and incomplete legislation, and shortcomings in regulations [9].
National Blood Legislation, Policy and Governance
Blood transfusion supports saving lives and improving health, but many patients in need of transfusion do not have timely access to safe, effective and quality-assured blood. Providing safe, effective and quality-assured blood should be an integral part of every country’s national health care policy and infrastructure.
WHO recommends [7] that all activities related to blood collection, testing, processing, storage and distribution – the manufacture – be coordinated at the national level through effective organization and integrated blood supply networks of independent blood establishments: a national blood system. This national blood system should be governed by a national blood policy [10] and legislative framework [11,12] to promote uniform implementation of current standards, consistency in the quality, safety and clinical efficacy of blood and blood products.
In 2018, 73 % or 125 out of 171 reporting countries mentioned they have a national blood policy [8]. However, only 66% or 113 out of 171, have a specific framework legislation covering the safety and quality of blood transfusion, largely in high-income countries, including:
- 79% of high-income countries (HICs)
- 63% of middle-income countries (MICs)
- 39% of low-income countries. (LICs)
Blood Supply
About 120 million blood donations are collected worldwide, 40% of these are collected in high-income countries, home to 16 % of the world’s population [8].
About 13,300 blood centers in 169 countries report collecting a total of 106 million donations. Collections at blood centers vary according to income group. In the low-income countries the median annual donations per blood center is 1,300, in lower-middle-income countries 4,400 and in upper-middle-income countries 9,300, as compared to 25,700 in high-income countries. Most of these blood centers are hospital-based and have insufficient economy-of-scale.
There is a marked difference in the level of access to blood between low- and high-income countries. The whole blood donation rate is an indicator for the general availability of blood in a country. The median blood donation rate in high-income countries is 31.5 donations per 1000 people. This compares with 16.4 donations per 1000 people in upper-middle-income countries, 6.6 donations per 1000 people in lower-middle-income countries, and 5.0 donations per 1000 people in low-income countries; 60 countries report collecting fewer than 10 donations per 1000 people. Of these, 34 countries are in the WHO African Region, four in the WHO Region of the Americas, four in the WHO Eastern Mediterranean region, four in the WHO European Region, five in the WHO South-Eastern Asia Region, and nine in the WHO Western Pacific Region [9]. All are low- or middle-income countries.
Many factors influence the requirements for blood to meet the health care needs of a population, as with all other treatment modalities. These include health care policies, income levels, current status and rate of development of the health care system, and accessibility of health care facilities to the public, all intimately related to the Universal Health Coverage (UHC) program and the seventeen Sustainable Development Goals (SDG) [13,14]. The need for, demand for, and use of blood in a country could be affected by geography, population migration, and epidemiology of diseases for which blood is needed but also competency of governance and stewardship, levels of knowledge acquirement, and transfusion medicine education and its environment. Therefore it is important to agree on definitions of need for, demand for, and use of blood [9] –
Need:
An estimation of the amount of blood needed to meet the transfusion requirements of the patient population according to current policies, clinical guidelines and best practices.
Demand:
The amount of blood that would be transfused if all prescriptions for blood were met. Demand may reflect appropriate or inappropriate indications and practices.
Use:
The actual amount of blood currently transfused; use may be appropriate or inappropriate.
Ideally there should be a balance between the demand and the need, translated into a demand–supply equilibrium based on appropriate and evidence-based use and demand-based manufacturing of collected units of blood; hence the two important interfaces to which the blood establishments are connected – clinical and societal. A model of need for, demand for, and use of blood in the LMIC is given in Figure 1.
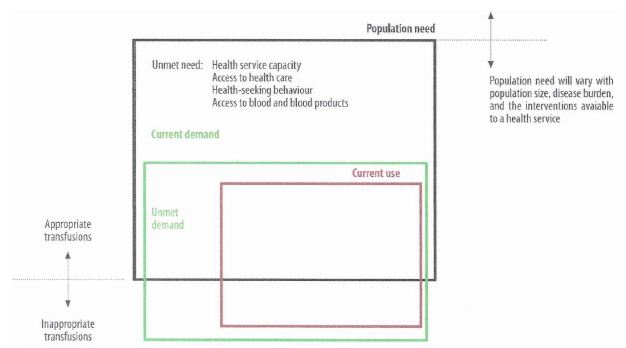
Figure 1: Paradigm or model of need for, demand for, and use of blood (9). Red = current use; green = unmet demand (in the box) and total (above the box); black = total population need. Below the black box = inappropriate demand and transfusion.
Need
Based on data reported by 157 countries to the Global Database on Blood Safety (GDBS) (9), 89% of whole blood donations collected globally were processed into components: 96% in high-income countries, 96% in upper-middle-income countries, but 75% in lower- middle-income countries, and only 38% in low-income countries. Across the six WHO regions, the percentages for processing blood into transfusable components were 42% in the African Region (AFR, 18/43 countries), 50% in the South-East Asia Region (SEAR, 5/10 countries), 57% in the Eastern Mediterranean Region (EMR, 8/14 countries), 60% in the Western Pacific Region (WPR, 12/20 countries), 71% in the Region of the Americas (AMR, 22/31 countries), and 95% in the European Region (EUR, 37/39 countries). These data provide a global picture of clinical blood component need and demand in the six WHO regions in the world.
Demand
Shortages of blood, whether real, f ictitious or potential, have impacted all countries at different times and periods, including more recently during the COVID-19 pandemic and ongoing humanitarian emergencies. In the early stages of the pandemic there were major concerns about lack of availability of blood for transfusion. Strategies and recommendations for responding to potential blood shortages must be incorporated into resilience planning for the blood supply by countries, and blood system and service operators and institutions [7,15,16].
Use
Data reported indicate significant differences in the age distribution of patients transfused. In high-income countries, the most frequently transfused patient group is aged over 60 years, which accounts for up to 76 % of all transfusions. In low-income countries, up to 54% of all transfusions are for children aged under 5 years (mostly malaria anemia), usually followed by females aged between 15 and 45 years (obstetrics). The WHO 2018 data [9] on distribution of units of blood transfused in different clinical departments in hospitals or other transfusion prescribing and performing health facilities from 19 countries in the African Region (AFR) revealed that among 2,248,721 units of blood were transfused; 466,625 (21%) were transfused to patients in pediatric departments, and 427,289 (19%) to patients in obstetrics and gynecology departments. In five of the 19 countries, more than 30% of blood was transfused to pediatric patients: Democratic Republic of the Congo 60%, Benin 58%, Burkina Faso 39%, Congo 33%, and Comoros 31%. Five countries reported that more than 30% of blood was transfused to gynecological and obstetric patients: Burkina Faso 61%, Cameroon 55%, Comoros 40%, Eswatini 32%, and Burundi 32%. Blood use for trauma and major bleeding varied considerably, although generally at lower rates than for patients in pediatrics, obstetrics and gynecology departments. The same data from the WHO AFR suggest that rates of usage in emergency and resuscitation departments in some countries approaches 23% to 34% (Madagascar 23%, Gabon 26%, Sao Tome and Principe 28%, and Cabo Verde 34%). Although these data indicate that children and women are the recipients who are most frequently transfused in low-income countries, it should be noted that these results are dependent on the accuracy of disease coding and documentation – e.g., it is possible that blood use in emergency departments is covered by surgery departments in some countries [9].
Another, often disrespected, observation is the almost daily occurring under transfusion and late transfusion due to poor logistics, organization and governance of the blood system, and poor to failing communication between prescribing and treating clinicians and blood manufacturers and suppliers. A number of studies have highlighted the burden of severe anemia in under 5 years of age children, often due to malaria. Mortality rates are significant, and deaths may occur within a few hours of arrival in hospital, indicating the importance of ambulance transport, access to timely blood transfusion support, which is often not available [15-19] and road conditions. Failure to recognize the presence of severe anemia resulted in lack of transfusion in some cases [20]. Currently, there are very limited data or studies available on unmet needs for blood transfusion in LMICs.
Consequences
So far, available and practicable knowledge seems a precious asset, because of the hampering and poorly organized Academic (tertiary) education and the lack of knowledge economy in most developing countries [21,22]. Consequence is not only the existence of a weak health care system but also the weakness of the supporting blood system. That leads to gaps in availability, safety, processing and clinical efficacy of blood transfusion in most LIMCs.
Unnecessary transfusions and unsafe transfusion practices expose patients to the risk of serious adverse transfusion events and transfusion-transmissible infections. Evidently, unnecessary transfusions also reduce the availability of blood and blood products for patients who are in need.
WHO recommends over the last decades the development of systems, such as hospital transfusion committees and hemovigilance [23], to monitor and improve the safety of transfusion processes. In this regard [9]:
- 128 countries have national guidelines on the appropriate clinical use of blood: 32 countries in the African region (74% of reporting countries in the region), 23 in the Americas Region (70%), 12 in the Eastern Mediterranean Region (67%), 33 in the Europe Region (80%), 9 in the South East Asia Region (90%), and 19 in the Western Pacific Region (76%). However, many of these guidelines have never been updated.
- Hospital transfusion committees (HTCs) are present in 48% of the hospitals performing transfusions: 62% in hospitals in high-income countries, 35% in upper-middle-income countries, 31% in lower-middle-income countries and 25% in low-income countries. However, many of these HTCs are dormant.
- Systems for reporting adverse transfusion events are present in only 55% of the hospitals performing transfusions: 74% in hospitals in high-income countries, 35% in upper-middle- income countries, 22% in lower-middle-income countries and 18% in low-income countries. However, these reporting systems are often stand alone and not embedded in a quality system and its management.
- Of reporting countries 49% have a hemovigilance The European Region, due to the European Union, has the highest percentage of countries with active hemovigilance systems (81%), followed by the Western Pacific Region (50%), the Eastern Mediterranean Region (50%), Africa Region (40%), South-East Asia Region (40%), and the Americas (21%), almost exclusively in North America and some in Latin America.
Other aspects such as manufacturing, cold chain and transportation have not been discussed as they represent the laboratory technical field of the vein-to-vein operations and are in fact the most easy and artisan parts of the chain.
Finally
There are remarkable epidemiological variations between countries and regions. The risk of transmission of serious infections, including HIV and hepatitis, through inadequately tested or even not tested unsafe blood, and existing chronic blood shortages brought global attention to the importance of blood safety and availability. With the goal of ensuring universal access to safe blood and blood products, WHO but also several professional associations and individual experts have been at the forefront to improve blood safety and availability, and recommend an integrated strategy for blood safety and availability [1- 3,7,10,24,25].
Lessons to Learn
WHO supports countries in developing national blood systems to ensure timely access to safe and sufficient supplies of blood and blood products and good transfusion practices to meet patient needs whether normal or emergency situations [7,20,21].
WHO provides policy guidance [10] and technical assistance [7,24-26] through development projects to countries for ensuring universal access to safe blood and blood products and works towards self-sufficiency in safe blood and blood products based on voluntary unpaid blood donations to achieve Universal Health Coverage (UHC). Unfortunately, that resulted in insufficient positive effects and often seems to be a bridge too far because of the shortage in knowledge and inadequate legislation and regulations.
To develop a sustainable and well-functioning national and integrated blood system that consistently meets the changing supportive needs of blood and blood components including the plasma-derived medicinal products (PDMPs), the principles should translate in knowledge and comprehension through a well- designed education system (education environment and teaching climate) anchored in a specific law and related regulatory mechanism [7,21].
There needs to be a governmental oversight and comprehension of what a blood system and Transfusion Medicine mean as integrated elements of the national health care system in the public health [7]. But also the development of stewardship, professionalism and a quality culture among the TM professionals, whether medical, nursing, laboratory or apprentice [27-29].
After all, blood transfusion is the most frequently practiced human transplant where Transfusion Medicine, after a long history, is now a fully accepted clinical discipline and science.
References
- Global Perspectives and Practices in Transfusion Eichbaum QG et al. eds. AABB Press, Besthesda MD 2023
- Global Education, Training and Staffing in Transfusion Eichbaum QG, et al. eds. AABB Press, Bethesda MD 2024
- WHA Resolution 72 ‘Utilization and Supply of Human Blood and Blood Products’, Geneva: World Health Assembly 1975. [Available at https: //www.who.int/blood. safety/en/WHA28.72.’df (accessed Jan, 24,2025)]
- WHA Resolution 12 ‘Availability, Safety and Quality of Blood Products’, Geneva: World Health Assembly 2010. [Available at https: //www.who.int/publications/i/ item/WHA63.12 (accessed Jan, 24,2025)]
- World Health Organization. Model List of Essential Medicines 23rd list 2023. [Available at http: // https: //www.who.int/publications/i/item/WHO-MHP-HPS- EML-2023.02 (accessed Jan. 24, 2025)]
- Guidelines on management of blood and blood components as essential medicines, Annex 3, TRS No 1004[Available at https: //www.who.int/publications/m/item/ blood-and-blood-components-as-essential-medicines-annex-3-trs-no-1004 {accessed Jan 25, 2025)]
- Action framework to advance universal access to safe, egffective and quality-assured blood products 2020-2023. Geneva: World Health Organization, 2020 Licence: CC BY-NC-SA 3.0 IGO
- Blood Safety and Key Facts. [Available at https: //www.who.int/news- room/fact-sheets/detail/blood-safety-and-availability (accessed Jan. 24, 2025)]
- Global Status Report on Blood Safety and Availability Geneva: World Health Organization, 2022. Licence: CC BY-NC-SA 3.0 IGO
- Aide Mémoire for National Health Policy Makers: Good Policy Process for Blood Safety and Availability. Geneva 2008 WHO/EHT/08.02
- Smit Sibinga CT (2017) Existing and recommended legislative framework for a National Blood Transfusion Glob J Transfus Med 2: 89-96.
- Assessment criteria for National Blood Regulatory Systems. World Health Organization 2012. WHO Press, Geneva
- Universal Health Coverage. [Available at https: //www.who.int/health-topics/ universal-health-coverage#tab=tab_1 (assessed Jan 24, 2025)
- United Nations. UN Sustainable Development Goals. New York, US 2016 [Available at htytps: //www.un.org/sustainabledevelopment/sustainable-development-goals/ (assessed Jan. 24, 2025)]
- Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, et (2020) Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematology 7: e756-e764. [Crosref]
- Guidance on ensuring a sufficient supply of safe blood and blood components during Geneva: World Health Organization, 2022. Licence: CC BY-NC-SA 3.0 IGO
- Kiguli S, Maitland K, George EC, Olupot-Olupot P, Opoka RO, et (2015) Anaemia and blood transfusion in African children presenting to hospital with severe febrile illness. BMC Medicine 13: 21-31. [Crossref]
- Thomas J, Ayieko P, Ogero M, et (2017) Blood transfusion delay and outcome in county hospitals in Kenya. American Journal of Tropical Medicine and Hygiene 96: 511-517.
- Cheema B, Molyneux EM, Emmanuel JC, et (2010) Development and evaluation of a new paediatric protocol for Africa. Transfusion Medicine 20: 140-151.
- WHO recommendations for the prevention of postpartum haemorrhage. Geneva: World Health Organization; 2012 [Available at: http: //apps.who.int/iris/bitstream/ handle/10665/75411/9789241548502_eng.pdf , (accessed Jan 24, 2025)].
- Louw VJ, Smit Sibinga CTh, Wessels P-L, Barrett CL, Rambiritch V (2024) Educational Systems, Environments and Opportunities for Change in Transfusion Medicine in LMICs. In: Eichbaum QG et al eds. Global Education, Training and Staffing in Transfusion Medicine .AABB Press MD 6: 125-152.
- Smit Sibinga CTh, Al Riyami AZ, Oladejo MA, Kajja I (2022) Poor Economics – Knowledge Economy and the Existing Knowledge Gaps (Higher and Academic Education) in How to Overcome? The Electronic Journal of Knowledge Management 20: 17-26.
- A guide to establish a national haemovigilance system. Geneva: World Health Organization 2026 WHO Press
- Guide to identify barriers in blood services using the blood system self-assessment (BSS) Geneva: World Health Organization, 2023. Licence: CC BY-NC-SA 3.0 IGO
- Web annex. Blood System sel-assessment. In: Guide to identify barriers in blood services using the blood system self-assessment (BSS) tool. Geneva: World Health Organization, 2023. Licence: CC BY-NC-SA 3.0 IGO
- Aide Mémoire for Ministries of Health: Developing a National Blood World Health Organization. 2011 WHO/EHT/11.1
- Smit Sibinga CTh, Rambiritch V, Louw VJ (2023) Transfusion Medsicine Education: A Global Model Focused on Professiionalism. In: Eichbaum QG et al. eds. Global Perspectives and Practices in Transfusion AABB Press Bethesda, MD 18: 505-528.
- Louw VJ, Al-Riyami AZ, Barrett CL, Johnson ST (2023) Integrated Approach to Transfusion Education for Clinicians, Nurses and Laboratory Professionals. In: Eichbaum QG et eds. Global Perspectives and Practices in Transfusion Medicine. AABB Press Bethesda, MD 19: 529-562.
- Smit Sibinga CTh, Hasan F (2020) Quality Management or the Need for a Quality Culture in Transfusion Glob J Transfus Med 5: 9-16.