Abstract
The Lithium-ion battery (LIB) has been utilized in many applications for thirty years, from personal electronics to electric vehicles. Many developments during the past decades resulted in high energy density and capacity in LIBs. However, battery safety still remains an issue alongside the continuous growth in the LIB market. Recent high-profile hazardous incidents gained considerable attention from the public as well as among researchers. This short review summarizes the recent efforts in improving LIB safety on three fronts: (1) materials advancement, (2) early monitoring and detection of thermal runaway events, and (3) fault diagnosis and fault-tolerance controls.
Keywords
lithium-ion battery; battery safety; battery management system; fault diagnosis; fault-tolerance control
Introduction
Lithium-Ion Battery (LIB) technology and the industry experienced rapid development in the past three decades, due to the advantages of LIBs over other energy storage systems, including: high energy density, strong stability, low maintenance, and low self-discharge. The LIB is the predominant power source in both consumer electronics and Electric Vehicles (EVs). The global market of LIBs (Figure 1) increased from $18.8 billion in 2014 to $28.5 billion in 2018. It is expected to increase by 11.1% (compound annual growth rate, CAGR) to $53.3 billion by 2024 [1]. The continuous growth in the global EV market from 2014 to 2024, at a rate of 21.1% (CAGR), is anticipated to contribute to the growth in the LIB market [2]. Although the LIB offers many advantages, safety still remains as an issue. According to a report issued by the Federal Aviation Administration (FAA), the number of air/airline incidents involving the battery – lithium-battery-induced smoke, fire, extreme heat, or explosion – increased from 9 to 50 incidents annually from 2014 to 2018 [3]. There are many reports about EV fire accidents caused by LIBs. For example, an all-electric compact car BYD e6 (BYD Auto company, China) caught fire after being hit by a Nissan GTR, an accident that led to the deaths of three passengers in Shenzhen, China, in May 2012 [4]. The battery pack of a Tesla Model S immediately caught fire after the driver hit debris on a highway in Washington State in 2013 [5]. Improving LIB safety is an important issue that requires research into new materials for the device, as well as structure optimization, and system design.
A typical EV battery is a three-level assembly: battery cell, battery module, and battery pack (Figure 2). The battery cell is a basic unit, consisting of an anode, a cathode, a separator, and liquid electrolyte. Multiple battery cells are connected and placed into a frame, called a battery module. Finally, the several battery modules are assembled into a battery pack, along with a control system, and system protection. The battery pack is a complete system that can be installed in an EV. Fires in a battery cell occur because of physical and electrical faults [6]. A chain reaction fire in the battery cells results in an explosion of the battery pack, called a cascading thermal runaway event. As mentioned in a review by Mauger et al., a gasoline fire can only be ignited when the gas tank’s air level is between 1.4 and 7.6%, and temperature is above 200oC [7]. The gas tank design of the vehicle ensures that gasoline cannot self-ignite in a tank. Unlike gasoline, the cascading thermal runaway event can happen in the LIB. This review summarizes the recent research efforts to improve LIB safety on three fronts: (1) materials advancement, (2) early monitoring and detection of thermal runaway events, and (3) fault diagnosis and fault-tolerance controls.
Current efforts in battery safety improvements
1 . Materials advancements
Cathode (positive electrode) material can be categorized into three groups, based on crystal geometry:
- Lamellar compounds (lithium cobalt oxide – LCO; lithium nickel oxide – LNO; lithium nickel cobalt aluminum oxide – NCA; and lithium nickel manganese cobalt oxide – NMC)
- Spinel lithium manganese oxide – LMO
- Olivine lithium iron phosphate – LFP
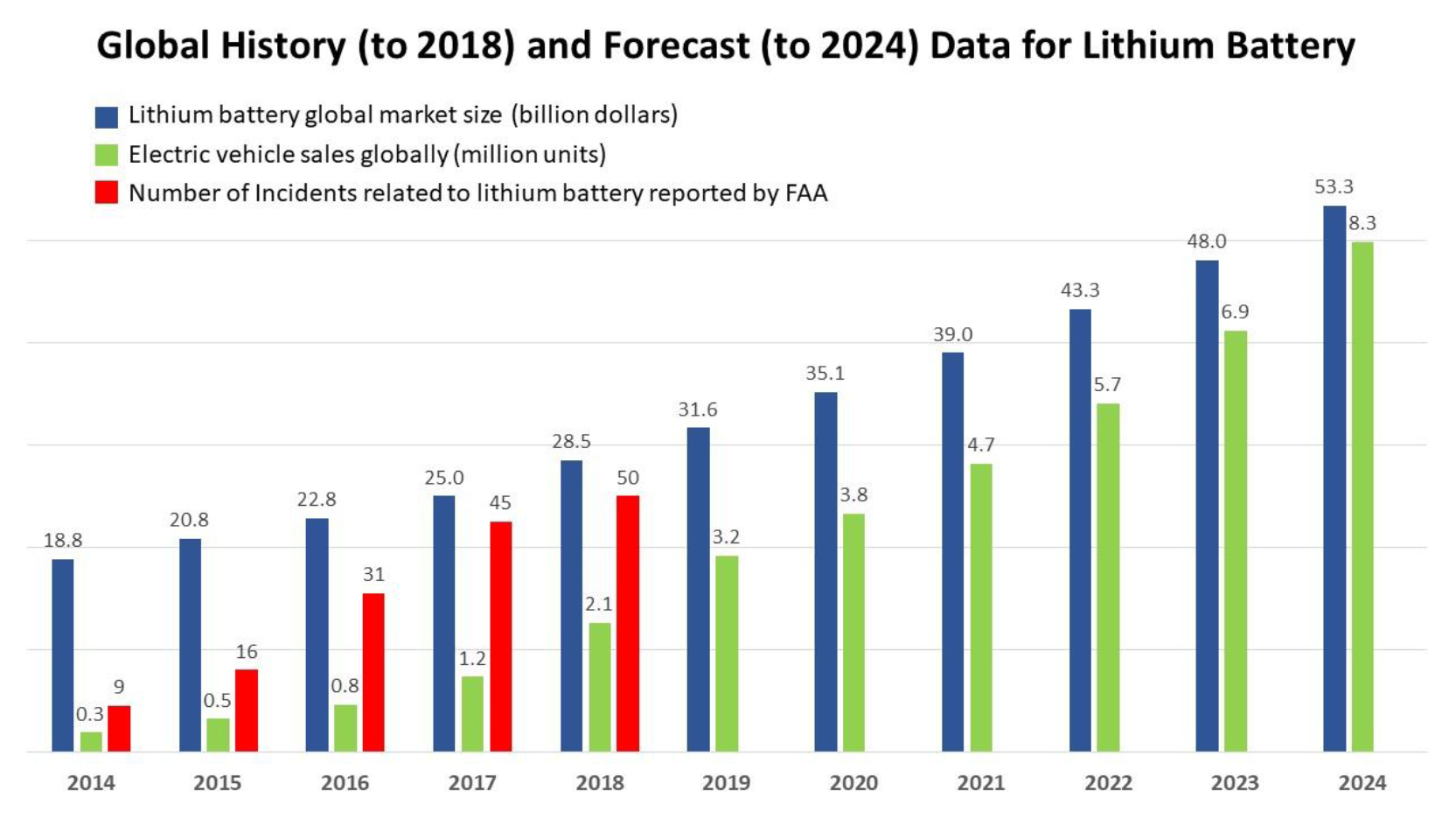
Figure 1. Global history and forecast data for lithium battery, including lithium battery global market size; electric vehicle sales globally; and numbers of problem incidents related to lithium battery reported by FAA [1–3].
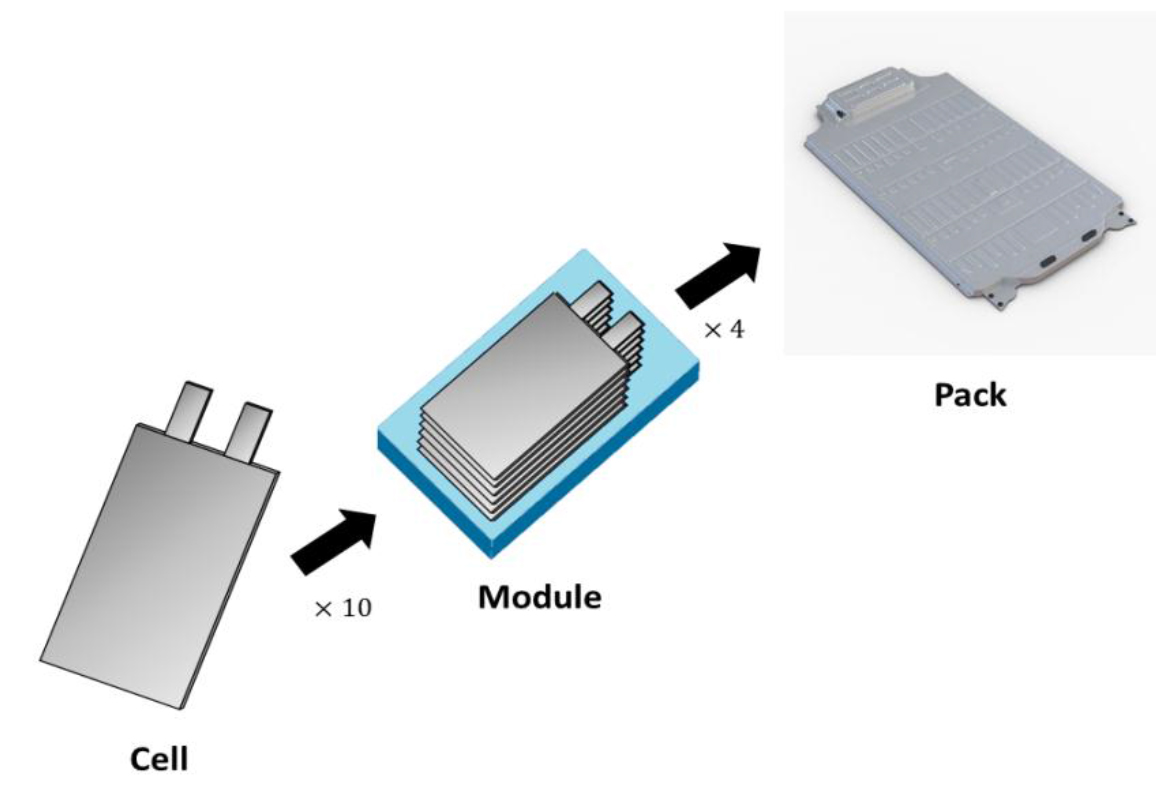
Figure 2. A typical battery pack assembly for EV.
LCO is the conventional cathode material used in the invention of LIBs by Sony. Because of materials shortages, cost, and the toxicity of cobalt (Co), transition metals (Mn and Ni) are used to partially substitute for Co. However, Huggins’ study showed that the partial pressure of oxygen at equilibrium varies exponentially, with a redox potential of the transition metal oxide vs lithium [8]. At 25oC, the equilibrium oxygen pressure for cathode materials is 1 atm, at a potential of about 3V. This oxygen pressure increases to greater than 50 atm at higher potentials. The lamellar compounds tend to lose oxygen (migrating to the counter-electrode, graphite) and produce carbon dioxide (exothermic reaction), which results in a thermal runaway and release of the emission gases.
LFP exhibits remarkable thermal stability because the oxygen is covalently bonded with the phosphorous atoms. Despite the fact that the operating voltage of the LFP with a graphite anode (3.2V) is lower than that of LCO (3.7–3.9V) and LMO (4.0V), the LFP cell passes the mechanical stress tests and short circuit tests without thermal runaway [7]. Hence, the LFP is commonly used for safety purposes in applications such as EVs and in industrial applications. Although the demand for the LFP cathode reached ~100 Gg (100,000 tons) in 2017, the forecast for LFP’s market share (along with LCO, NMC, NCA, and LMO) will decrease from 38% in 2017 to 15% in 2025 because of its low energy density [9]. Further developments of cathode materials with moderate to high energy density and safety are still needed.
Anode (negative electrode): spinel lithium titanate -LTO (theoretical capacity ~175 mAh/g) has been studied as an alternative to graphite (theoretical capacity ~372 mAh/g) [10–11]. Belharouak’s and Chen’s studies of lithiated LTO and lithiated graphite by differential scanning calorimetry showed that LTO exhibits higher exothermic onset temperature than that of graphite (130oC vs 100oC, respectively) and generates much less heat (383 J/g vs 2,750 J/g, respectively) [12–13]. In nail penetration tests, the temperature of the cells after penetration increased by only 5oC in a LIB with the LTO anode but rapidly increased by 130oC with the graphite anode [13]. Although the LTO has much lower theoretical capacity, it shows high thermal stability and capability to delay a thermal runaway event and may serve as a potentially safer alternative to graphite.
Separator is a porous polymeric membrane that prevents internal short-circuit events between the anode and cathode. Typical commercial separators are polypropylene PP (Tm ~165oC) and polyethylene PE (Tm ~130oC), configured as a single layer, or bi- and tri-layers [14]. Uniform and appropriate porosity (40–60%) is necessary to have uniform current density, retain a sufficient amount of liquid electrolyte, and maintain mechanical strength [15]. High porosity separators tend to shrink, which results in pore distortion and leads to an internal short circuit as the temperature increases. Zhang’s study showed that the mechanical properties of the PP and PE separators exhibited low punch strength and anisotropy in uniaxial tensile tests [16]. The separator failed easily under tension and punch. Recent separator improvements include surface coating of polydopamine (PDA), to inhibit Li-dendrite growth, and gamma irradiation, to enhance cross-linking of the PE polymer chains and enhance thermal stability of the PE separator, in order to improve safety [15].
Table 1. Typical materials in lithium-ion battery technology [7].
|
Cathode |
Anode |
Cell voltage (V) |
Energy density (Wh/kg) |
|
LiCoO2 (LCO) |
Graphite |
3.7–3.9 |
140 |
|
LiNiO2 (LNO) |
Graphite |
3.6 |
150 |
|
LiNi0.8Co0.15Al0.05O2 (NCA) |
Graphite |
3.65 |
130 |
|
LiNixMnyCo1-x-yO2 (NMC) |
Graphite |
3.8–4.0 |
170 |
|
LiMn2O4 (LMO) |
Graphite |
4.0 |
120 |
|
LiNi1/2Mn3/2O4 (LNM) |
Graphite |
4.8 |
140 |
|
LiFePO4 (LFP) |
Li4Ti5O12 (LTO) |
2.3–2.5 |
100 |
Liquid electrolyte includes ethylene carbonate (EC), diethyl carbonate (DEC), ethyl-methyl carbonate (EMC), and dimethyl carbonate (DMC). Different additives are added to improve safety, reduce gas generation, provide overcharge protection, and serve as fire-retardant. One drawback of liquid electrolyte is its flammable nature, due to the low flash point of each component. To overcome this hazard, ionic liquid (IL) has been added to reduce the flammability. However, the use of IL is limited in the commercial electrolyte because of its high cost. Current liquid electrolyte developments aim for low flammability, fire suppression, electrochemical stability, and performance. For examples, Zeng et al. reported a stable and electrode-compatible, non-flammable phosphate electrolyte by adjusting the Li salts-to-solvent molar ratio [17]. Wang et al. demonstrated that the concentrated electrolyte contains lithium salt and a flame-retardant solvent to suppress fires and permit stable charge-discharge cycling [18].
Solid-state electrolyte (SSE) is considered as a safe alternative to the organic liquid electrolyte and separator. SSE must demonstrate high ionic conductivity (greater than 10–4 S/cm at room temperature RT), negligible electron conductivity, and a broad electrochemical stability window [19]. There are three types of inorganic oxide-based SSE: a sodium super ion conductor (NASICON), with Li+ replacement; a garnet type; and a perovskite type. They have comparable bulk ionic conductivity (10–5-10–3 S/cm at RT) with the organic liquid electrolyte and show good chemical stability. However, there are several problems with the SSE: poor electrolyte/electrode interface in the NASICON-type; Li dendrite problems in the garnet type; and high interfacial resistance in the perovskite type. Sulfide-based SSE is another inorganic SSE with an ionic conductivity (10–2 S/cm) higher than that of the oxide-based SSE. However, it suffers from the generation of flammable hydrogen sulfide (H2S) upon exposure to the ambient atmosphere. The solid-state, hybrid electrolyte consists of a soft and flexible polymer electrolyte and a rigid inorganic SSE. It demonstrates good compatibility with the anode because of the intimate contacts. It is safer than the rigid inorganic SSE, because the uniform interfacial Li+ distribution also inhibits lithium dendrite formation [20]. Although the SSE has attractive properties, especially superior thermal stability and low flammability, problems such as lower bulk ionic conductivity at RT and interfacial mismatch between the solid-state electrolyte and the electrode materials still prevent widespread commercial application.
Beyond LIB: other battery chemistries – several non-lithium chemistries (Na, K, Mg, Ca, and Al) have been studied as potential alternative batteries to the LIB [21]. The Na+ and K+ in propylene carbonate (PC) exhibit higher mobility and ionic conductivity than that of Li+ because the Stoke’s radii in PC are in the order sequence of K+ < Na+ < Li+. Na-ion and K-ion batteries are expected to be a low-cost alternative to the LIB due to the abundance of Na and K resources, in addition to a similar energy density, similar battery design, and the same production process as the LIB [22]. Currently, studies of Na- and K-ion batteries and their safety are still in the development stage at research institutes.
2. Early monitoring and detection of thermal runaway events
Four different methods to monitor and detect thermal runaway events include (1) monitoring terminal voltage and surface temperatures, (2) an embedded optical fiber sensor, (3) electrochemical impedance analysis (EIS), and (4) a gas sensor monitor [23].
Terminal voltage and surface temperature monitoring method – This method uses the voltage and temperature sensors in real-time measurements of state of health (SOH), state of charge (SOC), and location of the faulty battery. Disadvantages of this method include high cost, low accuracy in thermal runaway prediction, and complexity of voltage sensor setup [23].
Embedded optical fiber sensor method – In this method, several types of fiber are used to set up optical sensors. For example, fiber Bragg grating arrays are attached on the surface of the cathode to record temperature and strain [24–26]. A nickel-coated fluorescent fiber is used for fluorescence lifetime measurements [23]. This technique can predict a thermal runaway event with high accuracy and directly monitor the internal temperature of the battery. However, the cost to set up the optical fibers and modify battery packaging is significant.
EIS method – The EIS technique uses an electrochemical impedance meter and a frequency response analyzer to determine the relationships between internal temperature and impedance phase shift or ohmic resistance [23, 27]. This method is able to predict the state of battery and thermal runaway temperatures with high accuracy. A drawback is the complex calibration process due to the fact that different battery systems have variant impedance parameters.
Gas sensing method – This simple and inexpensive method offers high accuracy and rapid detection, and functions by detecting vented gas concentration, because air flows faster than the speed of heat propagation in solid materials [23, 28]. Recently, Cai et al. validated the gas-sensing-based method by simulations [29]. In the simulation, the detection of the thermal runaway can be made at 85 seconds by the gas-sensing method, while the surface temperature measurement detected the thermal runaway propagation to neighboring cells at 710 seconds. Although the gas sensing-based method has many advantages, the sensor faults such as gas-sensor poisoning and gas cross-interference still persist.
3. Fault diagnosis and fault-tolerance control
A battery management system (BMS) consists of sensors, controllers, and computational algorithms. The BMS is designed to function in several ways: detect malfunctions and ensure battery safety, maintain accuracy and reliability, and predict and maximize battery life [30]. Battery faults are typically detected by data-driven approaches. Sensor faults are commonly diagnosed by model-based approaches, i.e. comparing the actual outputs to the estimated or nominal outputs (residual generation). A statistical cumulative-sum test is applied, rather than the selection of a fixed threshold, for high accuracy. The detected faults are then distinguished and monitored by fault-tolerant control (FTC) [31]. Most current and voltage sensors use Hall effect sensors and are usually subjected to bias and gain faults [32]. Many FTC methods for both the battery and sensors have been proposed and validated, including: re-arranging voltage measurement topology to distinguish between sensor and cell faults, without false detection and additional sensor [33–34]; nonlinear observability analysis for the sensor-biased fault-tolerance [35]; and active FTC to maintain battery temperature and deenergize the cell under faulty conditions [36].
Conclusion
Safety of LIBs has attracted considerable attention of researchers worldwide, as the incidence of LIB fires and explosions increases. SSE is a safe alternative but exhibits low ionic conductivity. Other battery chemistries have been studied but still remain in the development stage. BMS, the sensor system, and FTC are the most suitable measures to monitor and detect malfunctions of LIBs. Further developments of diagnostic schemes for fault detection and FTC for battery and sensors, together with novel materials developments, are needed to improve the safety of lithium-ion batteries.
References
- IMARC Group (2019) Lithium-ion Battery Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019–2024. Available at: https://www.researchandmarkets.com/reports/4775673/lithium-ion-battery-market-global-industry.
- Markets and Markets (2019) Electric Vehicle Market by Vehicle (Passenger Cars & Commercial Vehicles), Vehicle Class (Mid-priced & Luxury), Propulsion (BEV, PHEV & FCEV), EV Sales (OEMs/Models) Charging Station (Normal & Super) & Region – Global Forecast to 2030. Available at: https://www.marketsandmarkets.com/Market-Reports/electric-vehicle-market-209371461.html.
- FAA (2019) Lithium Batteries & Lithium Battery-powered Devices. Available at: https://www.faa.gov/hazmat/resources/lithium_batteries/media/Battery_incident_chart.pdf.
- China Autoweb (2012) Initial details on fiery crash involving BYD e6 that killed 3. Available at: https://www.greencarcongress.com/2012/05/bydcrash-20120528.html.
- Jensen C (2013) Tesla Says Car Fire Started in Battery. The New York Times. Available at: https://wheels.blogs.nytimes.com/2013/10/02/highway-fire-of-tesla-model-s-included-its-lithium-battery.
- Liu K, Liu Y, Lin D, Pei A, Cui Y (2018) Materials for lithium-ion battery safety. Sci Adv 4: 1–11.
- Mauger A, Julien CM (2017) Critical review on lithium-ion batteries: are they safe? Sustainable? Ionics (Kiel) 23: 1933–1947.
- Huggins RA (2013) Do you really want an unsafe battery? J Electrochem Soc 160: A3001-A3005.
- AVICENNE Energy. (2018) Current status and future trends of the global Li-ion battery market. Available at: https://www.charleshatchett.com/public/images/documents/2018/dr_christophe_pillot_current_status_and_future _trends_of_the_global_li-ion_battery_market.pdf.
- Writer B (2019) Anode Materials, SEI, Carbon, Graphite, Conductivity, Graphene, Reversible, Formation. In: Lithium-Ion Batteries, A Machine-Generated Summary of Current Research. Cham: Springer International Publishing,
- Tsai SY, Fung KZ, Ni CT (2015) Conductivity enhancement and thin-film processing of Li4Ti5O12(LTO) Spinel for Li battery applications. ECS Trans 68: 37–43.
- Belharouak I, Koenig GM, Amine K (2011) Electrochemistry and safety of Li4Ti5O12 and graphite anodes paired with LiMn2O4 for hybrid electric vehicle Li-ion battery applications. J Power Sources 196: 10344–10350.
- Chen Z, Belharouak I, Sun YK, Amine K (2013) Titanium-based anode materials for safe lithium-ion batteries. Adv Funct Mater 23: 956–969.
- Venugopal G, Moore J, Howard J, Pendalwar S (1999) Characterization of microporous separators for lithium-ion batteries. J Power Sources 77: 34–41.
- Lee H, Yanilmaz M, Toprakci O, Fu K, Zhang X (2014) A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy and Environmental Science 7: 3857–3886.
- Zhang X, Sahraei E, Wang K (2016) Deformation and failure characteristics of four types of lithium-ion battery separators. J Power Sources 327: 693–701.
- Zeng Z, Murugesan V, Han KS, Jiang X, Cao Y et al. (2018) Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat Energy 3: 674–681.
- Wang J, Yamada Y, Sodeyama K, Watanabe E, Takada K et al. (2018) Fire-extinguishing organic electrolytes for safe batteries. Nat Energy 3: 22–29.
- Zheng F, Kotobuki M, Song S, Lai MO, Lu L et al. (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389: 198–213.
- Liang J, Luo J, Sun Q, Yang X, Li R et al. (2019) Recent progress on solid-state hybrid electrolytes for solid-state lithium batteries. Energy Storage Mater 21: 308–334.
- Li M, Lu J, Chen Z, Amine K (2018) 30 Years of Lithium-Ion Batteries. Advanced Materials 30: 1800561.
- Kubota K, Dahbi M, Hosaka T, Kumakura S, Komaba S et al. (2018) Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion.” Chem Rec 18: 459–479.
- Liao Z, Zhang S, Li K, Zhang G, Habetler TG et al. (2019) A survey of methods for monitoring and detecting thermal runaway of lithium-ion batteries. J Power Sources 436: 226879.
- Meyer J, Nedjalkov A, Doering A, Angelmahr M, Schade W, et al. (2015) Fiber optical sensors for enhanced battery safety. Proc. of SPIE 9487: 94800Z.
- Fortier A, Tsao M, Williard ND, Xing Y, Pecht MG et al. (2017) Preliminary study on integration of fiber optic bragg grating sensors in li-ion batteries and in situ strain and temperature monitoring of battery cells. Energies 10: 838.
- Raghavan A, Kiesel P, Sommer LW, Schwartz J, Lochbaum A et al. (2017) Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 1: Cell embedding method and performance. J Power Sources 341: 466–473.
- Robinson JB, Darr JA, Eastwood DS, Hinds G, Lee PD et al. (2014) Non-uniform temperature distribution in Li-ion batteries during discharge – A combined thermal imaging, X-ray micro-tomography and electrochemical impedance approach. J Power Sources 252: 51–57.
- Wenger M, Waller R, Lorentz VRH, Marz M, Herold M et al. (2014) Investigation of gas sensing in large lithium-ion battery systems for early fault detection and safety improvement. In: IECON Proceedings 2014:5654–5659.
- Cai T, Stefanopoulou AG, Siegel JB (2019) Early Detection for Li-Ion Batteries Thermal Runaway Based on Gas Sensing. ECS Trans 89: 85–97.
- Omariba ZB, Zhang L, Sun D (2018) Review on health management system for lithium-ion batteries of electric vehicles. Electron 7: 72.
- Xiong R, Yu Q, Shen W (2018) Review on sensors fault diagnosis and fault-tolerant techniques for lithium ion batteries in electric vehicles. In: Proceedings of the 13th IEEE Conference on Industrial Electronics and Applications 2018: 406–410.
- Liu Z, He H (2015) Model-based sensor fault diagnosis of a lithium-ion battery in electric vehicles. Energies 8: 6509–6527.
- Xia B, Mi C (2016) A fault-tolerant voltage measurement method for series connected battery packs. J Power Sources 308: 83–96.
- Xia B, Nguyen T, Yang J, Mi C (2016) The improved interleaved voltage measurement method for series connected battery packs. J Power Sources 334: 12–22.
- Zhao S, Duncan SR, Howey DA (2017) Observability Analysis and State Estimation of Lithium-Ion Batteries in the Presence of Sensor Biases. IEEE Trans Control Syst Technol 25: 326–333.
- Dey S, Shi Y, Khanra M, Smith K (2019) Safer Batteries via Active Fault Tolerant Control. Am Control Conf 2019:1561–1566.