Abstract
Objective: Hyperglycaemia and diabetic complication during pregnancy interfered with birth defect and traditional usage of phytotherapy take a great attention. The present study searched for illustrate the interference of maternal diabetes on the development of exocrine pancreas during in utero growth and role of ginger extract in improving the disease.
Research design and methods: Sixteen pregnant rats were used and arranged into four groups (n = 4); control, ginger extract group, diabetes, diabetes and ginger supplementation. Diabetes was induced by single i.p. injection of streptozotocin (60 mg/kg in citrate buffer pH 4.5 plus 100mg/kg nicotinamide). Ginger watery extract (200mg/kg body weight) was orally daily supplemented from 5th day of gestation until 14th day of gestation. Pregnant were sacrificed at 14th day of gestation, fetuses were separated and exocrine pancreas were separated and fixed in either 10% phosphate buffered formalin or 2.5 cacodylate buffered glutaraldhyde for transmission electron microscopy. Immunohistochemistry of caspase 3 was carried out. During the gestation period, blood glucose level was measured in the studied groups.
Results: The present results revealed that diabetes developed atrophy and degeneration of the acinar epithelium of the pancreas. At ultrastructural level, there was a considerable missing of the secretory granules and arranged of rough endoplasmic reticulum taking the characteristic feature of fibrosis. Immunohistochemical analysis illustrated over expression of caspase 3 in acina epithelium. However, ginger water extract supplementation to diabetic mother improved the blood glucose level and histo-cytlogical and immunohistochemical picture manifesting marked alleviation of the drastic alterations compared to the control.
Conclusion: The author concluded that ginger extract showed a potent antioxidant activity, decreasing the diabetes associated oxidative stress and improved the fetal exocrine pancreas histo-cytological structure.
Introduction
The pancreas is composed of exocrine and endocrine portion. It is developed from the dorsal and ventral endodermal as a result of the epithelio-mesenchymal interactions. Exocrine acinar cells, are responsible for secretion of zymogens and digestive enzymes through a ductal network leading to the duodenum and facilitated digestion [1, 2]. Acinar cells builds up the exocrine pancreas and secrete up to more than 20 kinds of enzymes, such as proteinase, DNAses and lipases, which are important elements for digestion [3].
The knowledge of type T3c diabetes mellitus (T3cDM, MODY) is still unclear and involved in the impairing of exocrine pancreas, the reservoir of digestive enzymes. Little knowledge about the mark differences between T2DM and T3cDM. T3cDM is developed in early childhood growth [4] and characterized by chronic pancreatitis, pancreatic cancer. Inflammation, fibrosis leading to damage of both endocrine and exocrine function impairing insulin/glucagon secretion and dysfunction of pancreatic enzyme [5]. The prevalence of exocrine dysfunction in type-1 and type-2 diabetic patients reached approximately to 37.7% and 26.2% respectively [6]. Also, the exocrine pancreas dysfunction related to diabetes affects 1.8% of adults with new-onset diabetes. Cell death of the islet of Langerhans alters the secretion of insulin, glucagon, and pancreatic polypeptide resulting fluctuations in glucose levels. Diabetes induced acute or chronic pancreatitis, associated with co-existing cystic fibrosis or hemochromatosis [7,8].
At the same time, there is a great attention to the consumption of traditional medicine. Ginger (Zingiber officinale) is a rhizome popular food spice and showed varieties of bioactive components such as vallinoids, viz. gingerol[9] and paradol, shogaols, zingerone, and galanals A and B [10]. Its administration resulted in improvements of diabetic patients [11–13] and experimental animals [14,15] through managing of hyperglycemia glycemia, oxidative stress, and inflammation.
There was no available work concerned the influence of maternal diabetes on the developing of exocrine pancreas during intrauterine life. The present work searched for illustrated the light and transmission electron microscopically study of fetal exocrine pancreas of diabetic mother and the potential medical phytotherapy of watery ginger extract.
Materials and Methods
1. Applied dose of ginger watery extract
A known weight of dried ginger (Zingiber officinale) was mixed thoroughly with hot water weekly, filtrate and allows standing at room temperature. Each pregnant received 0.5 cc containing 200mg of the extract / kg body weight. Each dose was orally administered daily from 6th day of gestation till 16th day of gestation.
2. Induction of diabetes
Experimental type 2 diabetes mellitus was induced in all the rats by a single interperitoneal injection of streptozotocin (60 mg/kg) in citrate buffer (0.05 M) (pH 4.5) and 100mg/kg nicotinamide [16]. Hyperglycemia was verified by measuring the blood glucose within 240- 280 mg/dl were selected for the study.
3. Experimental animal work
Twenty fertile male and virgin female of albino rats (Rattus norvegicus) (at a ratio of 1 male to 3 females) weighing approximately 200g body weight, obtained from Hellwan Breeding Farm, Ministry of Health, Egypt and used for experimentation. They were housed in good ventilation on a 12-h light and dark cycle. Females were mated (1 male/3 females) overnight and zero dates of gestation were determined the next morning by observing the sperm in vaginal smear. The pregnant were arranged into four groups (n = 4 per each); control, ginger watery extract, diabetes, diabetes and ginger extract. Experimental work was carried out from zero date of gestation till 16th day intra-uterine growth. Water was allowed ad Libitum. At the end of treatment, they were sacrificed by light diethyl ether anesthesia and dissected at 14th days prenatal. Maternal blood was collected and serum was separated and kept in refrigerator. Also, the pregnant were dissected and their fetuses were separated and processed for the following investigations:
4. Biochemical investigations:
Serum levels of total cholesterol (TC) [17], Triglycerides (TG) [18] and high density lipoproteins (HDL) [19] were determined. In case of low density lipoproteins (LDL), it was calculated from the total concentrations of cholesterol (TC), HDL-cholesterol and triglycerides according to Friedewald et al [20]. The glucose regularly measured by blood glucometers one touch ultra (Life Scan Milipitas, CA, USA).
5. Histological investigation:
Pancreas of 14th day-old fetuses of the studied groups were separated and immediately fixed in 10% phosphate buffered formalin (pH 7.4), dehydrated in ascending grades of ethyl alcohol, cleared in xylene and mounted in molten paraplast at 58–62ºC. Five μm histological sections were stained with hematoxylin & eosin and investigated under a bright field light microscope.
6. Transmission electron microscopy
Specimen of pancreas of 16th day old fetuses of the studied groups were immediately fixed in 2.5 % glutaraldehyde in 0.1M cacodylate buffer (pH 7.4) and post-fixed in 1% osmium tetraoxide, dehydrated in ascending grades of ethyl alcohol, and embedded in epoxy–resin. Ultrathin sections were cut with a diamond knife on a LKB Ultratome IV (LKB Instruments, Bromma, Sweden) and mounted on grids, stained with uranyl acetate and lead citrate, and examined under a Joel 100CX transmission electron microscope (Musashino 3-chome, Akishima, Tokyo 196–8558, Japan).
7. Immunohistochemistry for caspase 3
Deparaffinized histological 5μm thick sections of pancreas of 16 day old fetuses were cut and mounted onto super frost plus glass slides (Fisher Thermo Scientific, Nepean, Ontario, Canada). At a normal room temperature, the tissue sections were processed for antigen retrieval by digestion in 0.05 % trypsin (pH 7.8) for 15 min at 37°C and incubated with the antibodies against caspase 3 (dilution 1:100 Thermo Fisher Scientific, Fremont, CA, USA; Cat. No. A1–70007) for overnight at 4°C, followed by treatment with a horseradish peroxidase streptavidin detection system (Dako), and DAB plus Chromagen for developing the immunoactivity. The immunohistochemical stained slides were counterstained with hematoxylin. Negative control was carried out by incubating slides with 1% non-immune serum phosphate buffer solution (PBS) solution. Specimens were investigated under a Leica BM5000 microscope (Leica Microsystems, Wetzlar, Germany) and photographed.
For assessments of image analysis, slides were photographed using Olympus® digital camera installed on Olympus® microscope with 1/2 X photo adaptor, using 40 X objective. The result images were analyzed on Intel® Core I5® based computer using Video Test morphology® software (Russia) with a specific built-in routine for area, % area measurement and object counting.
Results
Biochemical observations
Ginger-extract supplementation improved the diabetic associated increase of serum glucose level, LDL, triglycerides and total cholesterol levels (Fig.1).
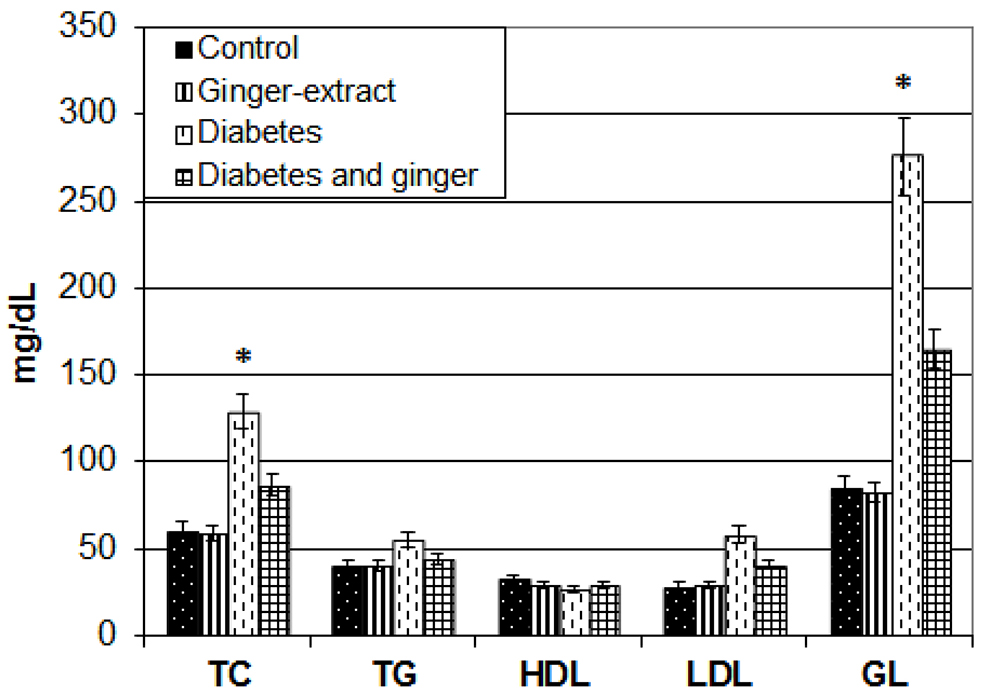
Figure 1. Chart illustrating serum glucose, LDL, TG, HDL and total cholesterol levels. Each result represent the mean ± SD of n = 5. * Significant at P < 0.05. Abbreviations; GL, glucose, HDL, high density lipoprotein; LDL, low density lipoprotein; TC, Total cholesterol.
Exocrine pancreas of 14th day fetuses
Fetuses of control and ginger-extract-supplemented mother exhibited normal structural pattern of exocrine pancreas. It is composed of tubuloacinar glands containing numerous acinar units. Each composed of pyramidal acinar cells with centrally located nuclei and basophilic cytoplasm surrounding their lumen (Fig. 2 A&B)
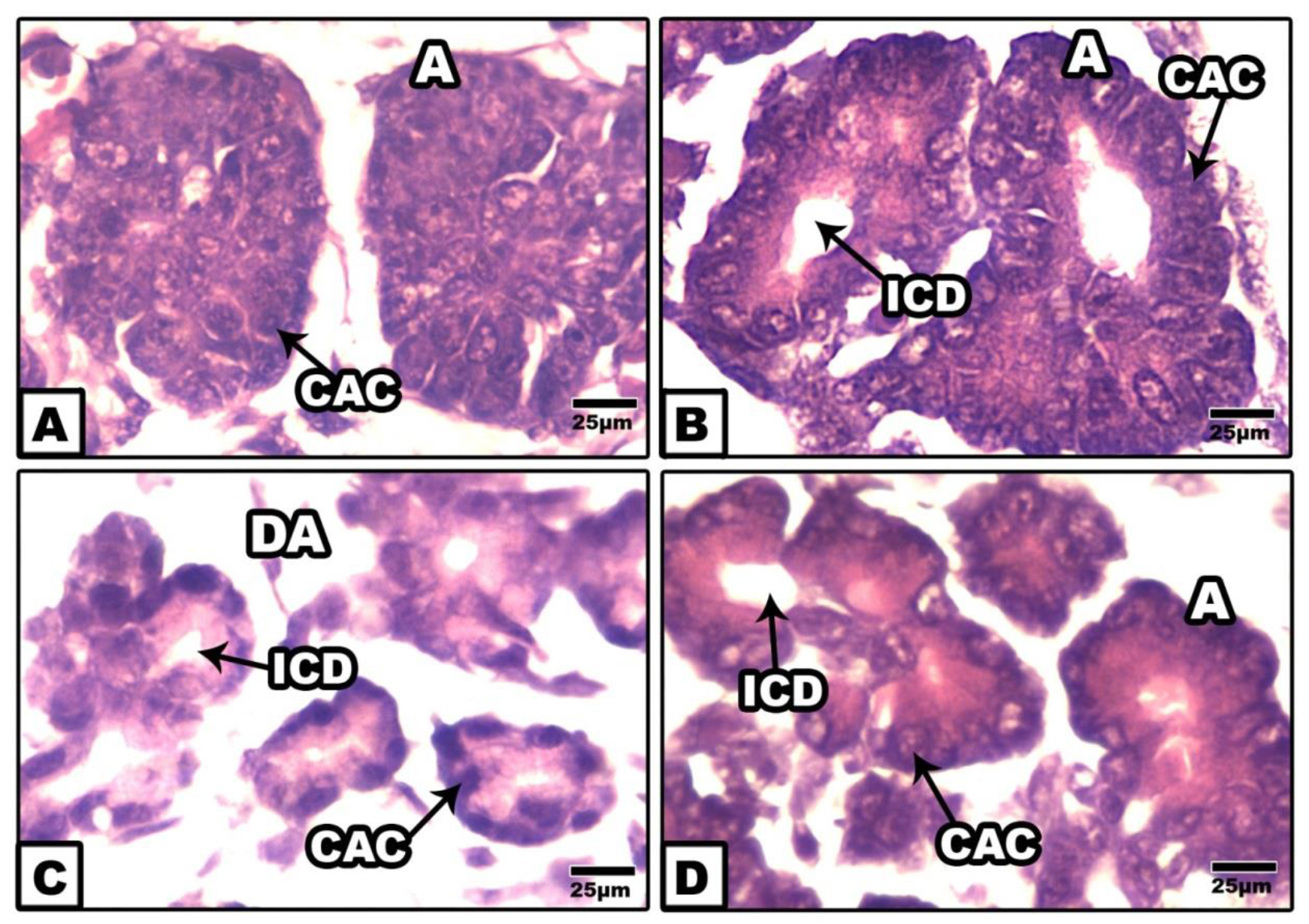
Figure 2. Photomicrographs of histological sections of exocrine pancreas of 14th day rat fetuses. A. Control. B. Cinnamon-ginger extract. Note the normal structural pattern of centro-acinar cells and intercalated duct. C. Foetuses of diabetic mother showing disorganized acinus with collapsed duct and damaged acinar lining cells. D. Foetuses of diabetic mother supplemented ginger extract supplementation showing improved cento-acinar cells and intercalated duct. HE. Abbreviations; A, acini, CAC, centro-acinar cell; ICD, intercalated duct ; DA,deformed acinar.
However, those of diabetic mother exhibited massive damaging of the acinar units and atrophied lumina. The acinar lining cells appeared swollen with pyknotic nuclei and eosinophilic cytoplasm. Interlobular ducts were lined with flattened epithelium (Fig.2C).
On the other hand, fetuses of diabetic mother supplemented ginger-extract revealed improvement of the pancreatic structure but of less developed in comparison with the control (Fig.2D).
At ultrastructural level, the exocrine acinar cells possessed normal cytological structure in control and ginger –treatment. Each exocrine cell possessed centrally located nuclei with peripheral heterochromatin and abundant euchromatin. The cytoplasm contained abundant electron-dense and faint spherical zymogen granules of varying sizes and shape. The granules were membrane-bound. Numerous mitochondria and rough endoplasmic reticulum were detected in-between the secretory granules. Glycogen granules were observed in the cytoplasm of the acinar cells. Blood vessels are detected around the acini and ducts (Figs. 3 A&B).
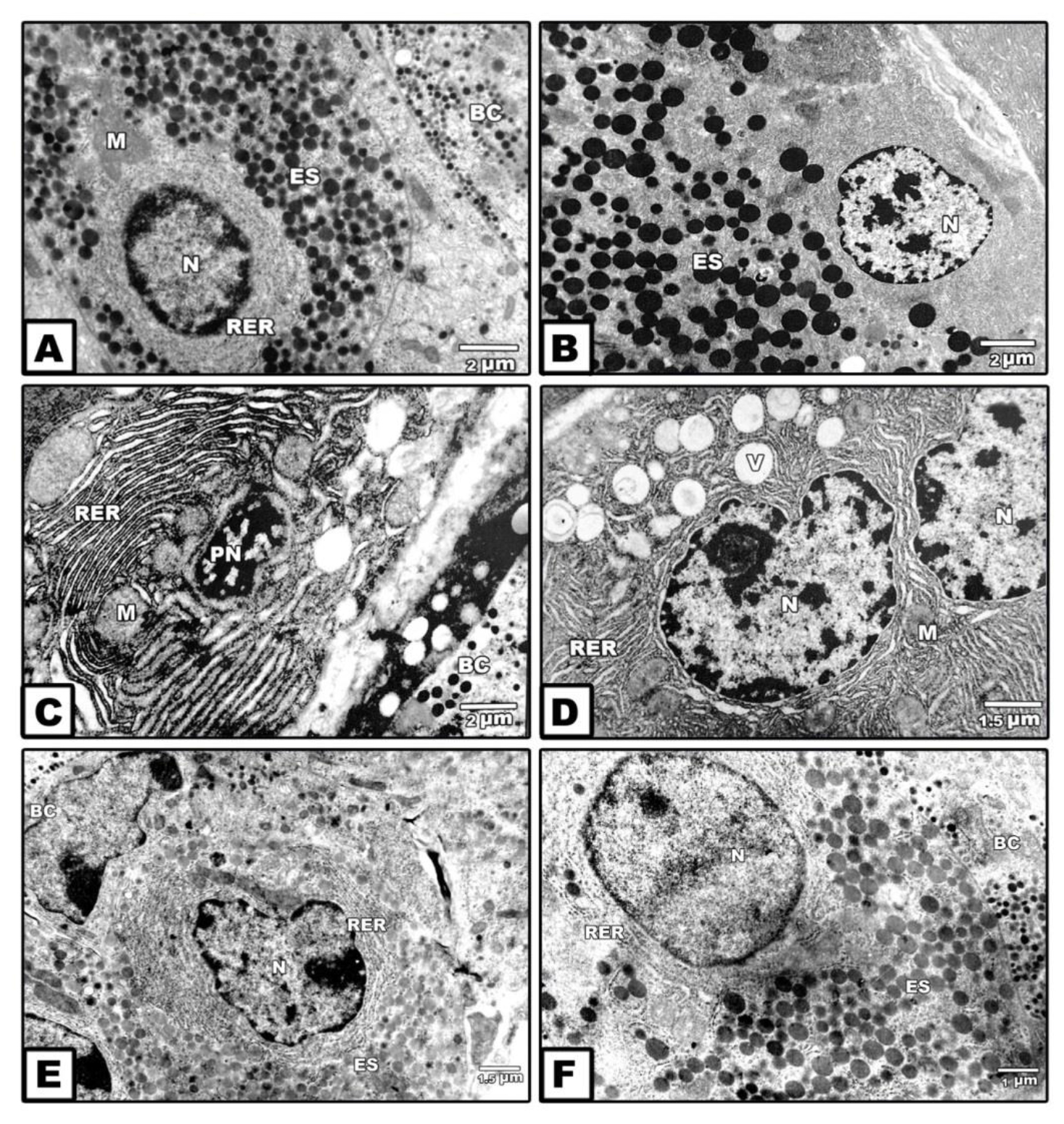
Figure 3. Transmission electron micrographs of exocrine pancreas of 14th day-old fetuses. A. Control. B. Maternally supplemented ginger extract. Note the presence of normal oval-shaped nuclei with peripheral marginal heterochromatin and central euchromatin. The is rich of electron –dense zymogen granules of different sizes containing in between mitochondria. C&D. Maternally diabetic showing apoptic atrophied nuclei composed electron-dense heterochromatin and surrounded by a network of clumped rough endoplasmic reticulum containing in between atrophied mitochondria (C). Another specimens illustrates nuclei with irregular nuclear envelope, lipid vacuoles and missing of secretory granules (D). E&F. Foetuses of diabetic mother supplemented ginger extract revealing increase secretion of light and electron-dense zymogen granules. Abbreviations; BC, blood capillary; ES, electron-dense-secretory granule; M, mitochondria, N, nuclei; PN, pyknotic nuclei; RER, rough endoplasmic reticulum; V, lipid vacuoles.
In those maternally diabetic, the acinar cells exhibited apoptic nuclei composed of electron-dense heterochromatin and surrounded by a network of clumped rough endoplasmic reticulum enclosed in between atrophied mitochondria manifesting fibrotic structure. In some other cells, the nuclei outlined by irregular nuclear envelope and their cytoplasm contained abundant lipidvacuoles of varying sizes and missing of their inclusion of secretory granules (Figs. 3 C&D).
On the other hand, fetuses of diabetic mother supplemented ginger extract restored the presence of faint secretory granules was comparatively decreased in some cells and increased in other ones. The criteria structure of the acinar cells is improved but less developed compared to the control (Figs. 3 E&F).
Following immunohistochemical staining with caspase 3, the fetuses of diabetic mother exhibited overexpression of dark brown reaction in acinar cells (Fig. 4C) in comparison with the negative staining affinity in control and ginger supplementation (Figs. 4 A&B). Fetuses of diabetic mother supplemented ginger extract revealed marked reduction of the immunohistochemical reaction (Fig. 4D). Image analysis revealed increased regional area of immunohistochemical reaction in acinar cells of diabetic mother compared to the other studied groups (Fig. 5).
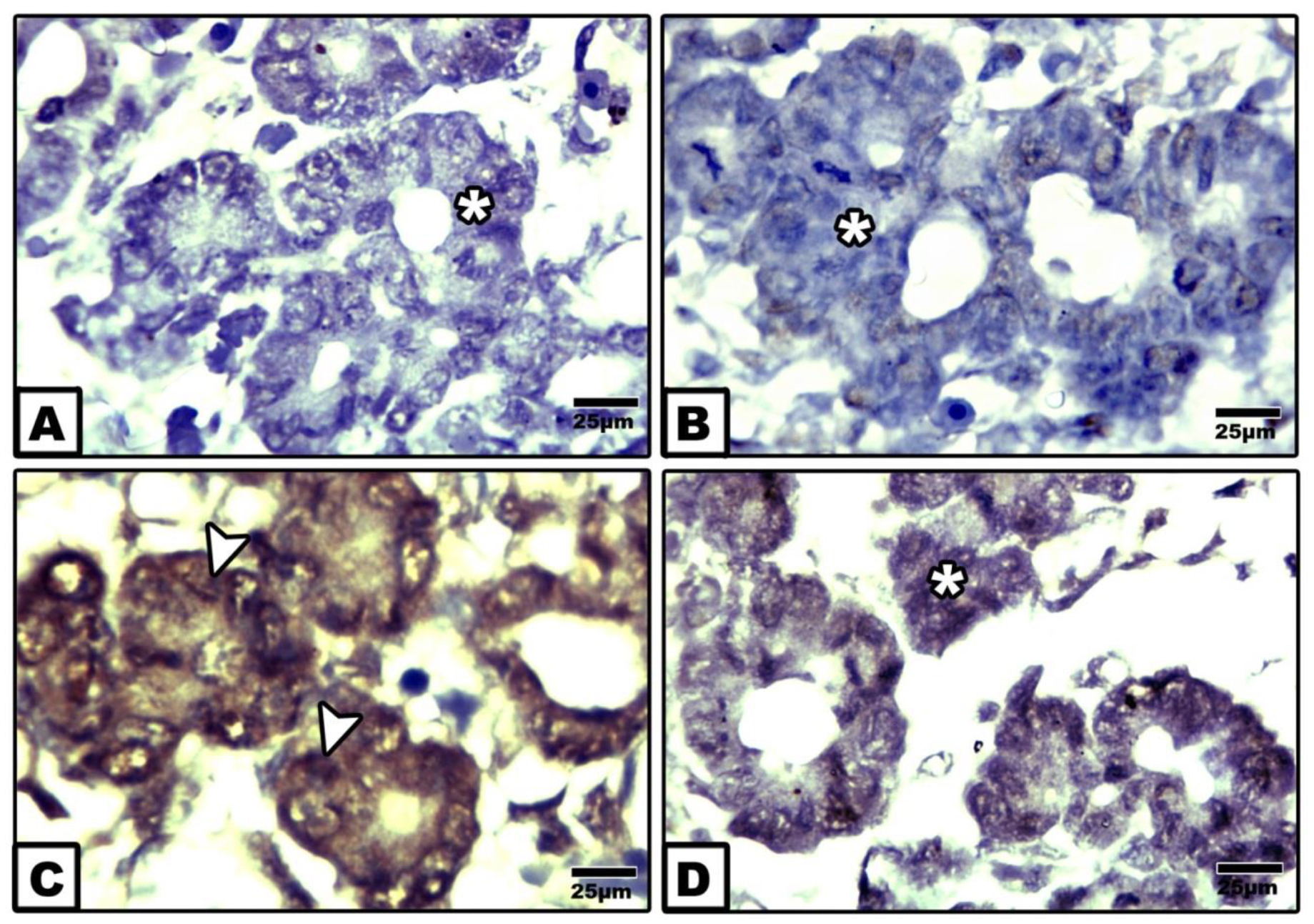
Figure 4. Photomicrographs of formalin fixed histological section of exocrine pancreas of 14th day-old fetuses maternally immunohistochemically stained with caspase 3. A. Control. B. Ginger extract-treatment. Note negative immuphistochemically stained with caspase 3. C. Maternally diabetic showing increased immunohistochemical affinity in acinar cells. D. Ginger extract showing marked reduction of the expression of caspase 3.
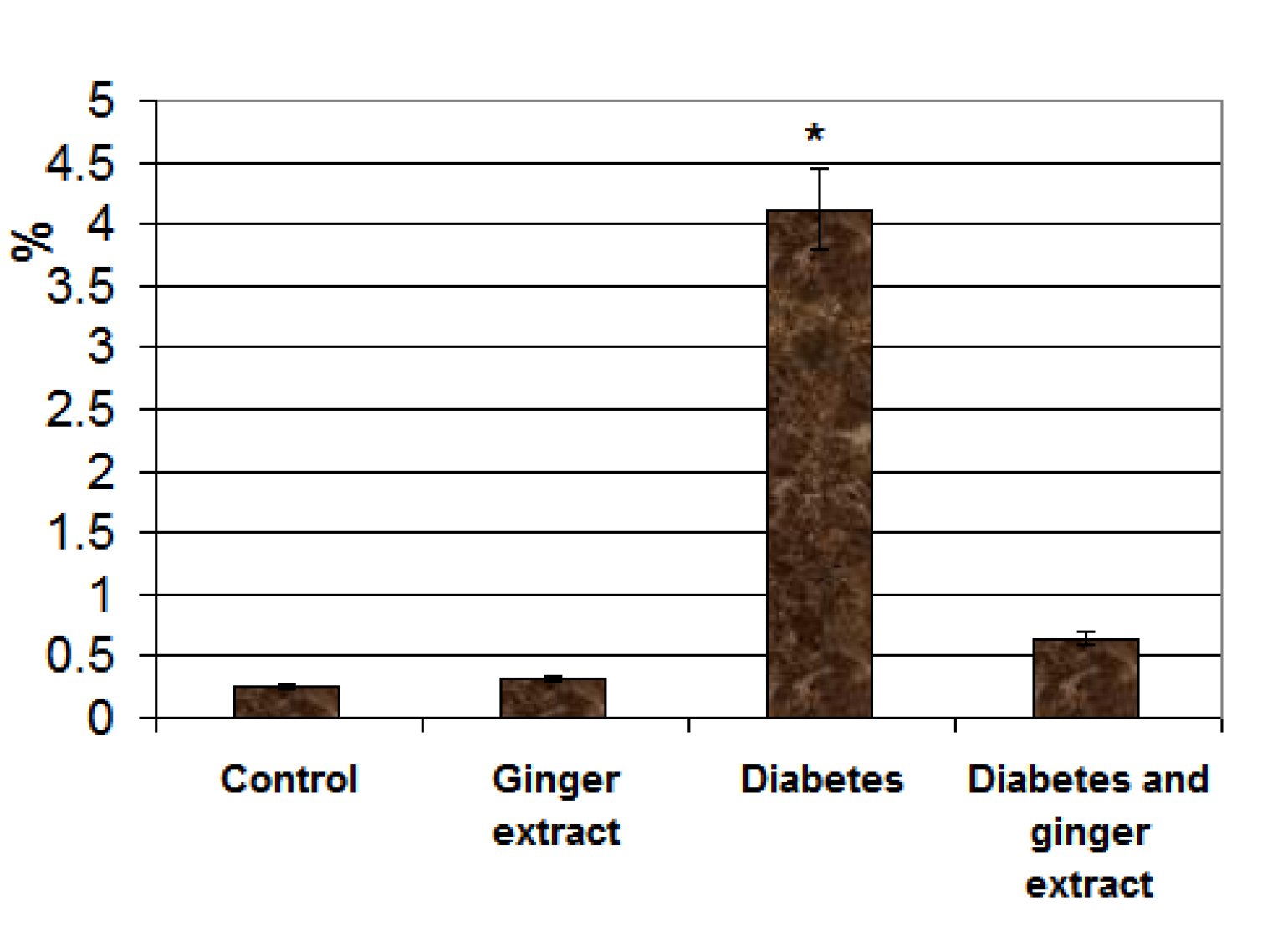
Figure 5. Chart illustrating image analysis of surface immunohistochemical reactive areas with caspase 3 of acinar cells of fetuses of diabetic mother compared to the other studied groups. Each result represent the mean ± SD of n = 5. Star means significant at P < 0.05.
Discussion
The present findings revealed that 14th day-old fetuses of diabetic mother exhibited abnormal histological structure of exocrine pancreatic cells including eosinophilia cytoplasm and pyknotic nuclei of acinar cells and almost missing of their lumina. Although there is no detected the presence of inflammatory cells, other authors revealed that inflammation may be occurred during organogenesis of the lymphopoietic-hematopoietic system, based on hematopoietic cell-mesenchymal cell interactions [21].
The presence of zymogen granules in the acinar cells has been reported by Conklin [22] and Laitio et al. [23] in human fetal pancreas. Tadokoro et al. [24] reported the presence of increased number and density of zymogen granules before birth in rats. Inagaki et al. [25] mentioned that the secretory granules are responsible for stored zymogen granucles and secretion of lipase, trypsin, and amylase in fetal acinar cells. Rough endoplasmic reticulum and the Golgi apparatus are involved in synthesis of zymogen granule in the adult [26].
At ultrastructure level, the observed findings reported a detected missing of the secretory granules and increased network of endoplasmic reticulum surrounding the apoptic nuclei forming the fibrotic structure. Lipid vacuoles of varying sizes were distributed in the cytoplasm. Also, atrophied mitochondria with damaging internal structure were observed in comparison with the control.
Similar findings of reduced secretory granules were reported in E2F1/E2F2 compound-mutant mice develop non-autoimmune insulin-deficient diabetes [27].
Diabetes may involvein gene defects leads to pancreatic lipomatosis and exocrine pancreatic insufficiency in childhood, and progressed till 34 years [28]. The observed damage of both mitochondria and rough endoplasmic reticulum facilitated accumulation misfolding protein with intracellular and extracellular aggregation exerting a cytotoxic effect and caused progression of disease of the islets of Langerhans [29].
The apparent reduction and number of mitochondria and deposition of fat vacuoles reflected the depletion of energy level of acinar cells and consequently loss of pancreatic function.
Similar findings were reported by Kaido et al. [30] in a murine model of primary carnitine deficiency.
Also, the present study reported the development of congenital malformation of acute pancreatitis in fetuses of diabetic mother. Damaging of the acinar cells led to depletion of the majority of enzymes needed for digestion.
Pin et al. [31] reported that the acinar cells of the exocrine pancreas is responsible for the development of up >90% of the cells within the pancreas especially β cells. The conversion of acinar cells to either β cells involves alterations in the expression and activity of the transcription factor, and changes in their epigenetic program.
For my opinion in utero poor control of diabetes associated damage of the exocrine pancreas may be increased cell programming and increased the risk susceptibility for the developmental origin of diabetes during adult hood.
At the same time supplementation of ginger to diabetic mother ameliorated the structural pattern of the acinar cells, partially restoring the secretory granules and cell cytoskeletal structure.
Ginger was found to contain several bio components such as gingerol [9] and shogaols and zingerone [10] which improve hyperglycaemia and resoluted oxidative stress and inflammation. This led to decrease the programming of cell death and managing cell growth and differentiation [11,14, 15] .
The authors finally concluded that ginger supplementation during pregnancy may help to improve the antioxidant activity of the pancreatic cells and sustained growth and differentiation against the cytotoxicity of diabetes.
References
- Edlund H (2001) Developmental biology of the pancreas. Diabetes 50: 5–9.
- Zhou Q, Melton DA (2018) Pancreas regeneration. Nature 557: 351–358.
- Zhou Q, Law AC, Rajagopal J, Anderson WJ and Gray PA et al (2007) A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13:103–14.
- Fajans SS, Bell GI, Polonsky KS (2001) Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 345:971–980.
- Bhattamisra SK, Siang TC, Rong CY, Annan NC, Sean EHY et al (2019) Type-3c Diabetes Mellitus, Diabetes of Exocrine Pancreas – An Update. Curr Diabetes Rev.
- Talukdar R, Reddy DN (2017) Pancreatic Exocrine Insufficiency in Type 1 and 2 Diabetes: Therapeutic Implications. J Assoc Physicians India 65: 64–70.
- Bonnet-Serrano F, Diedisheim M, Mallone R, Larger E (2018) Decreased α-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: An analysis based on the nPOD repository. PLoS One 13: 0191528.
- Wynne K, Devereaux B, Dornhorst A (2019) Diabetes of the exocrine pancreas. J GastroenterolHepatol 34: 346–354.
- Kaul PN, Joshi BS (2001) Alternative medicine: Herbal drugs and their critical appraisal-part II. Prog Drug Res 57:1–75.
- Shukla Y, Singh M (2007) Cancer preventive properties of ginger: a brief review. Food ChemToxicol 45:683–690.
- Suk S, Kwon GT, Lee E, Jang WJ, Yang H et al (2017) Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice. MolNutr Food Res 61.
- Wang J, Ke W, Bao R, Hu X, Chen F (2017) Beneficial effects of ginger Zingiberofficinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci 1398: 83–98.
- Wang Y, Yu H, Zhang X, Feng Q, Guo X et al (2017) Evaluation of daily ginger consumption for the prevention of chronic diseases in adults: A cross-sectional study. Nutrition 36: 79–84.
- Ilkhanizadeh B, Shirpoor A, Khadem Ansari MH, Nemati S, Rasmi Y (2016) Protective effects of ginger (Zingiberofficinale) extract against diabetes-induced heart abnormality in rats. DiabetesMetab J 40: 46–53.
- de Las Heras N, Valero-Muñoz M, Martín-Fernández B, Ballesteros S, López-Farré A (2017) Molecular factors involved in the hypolipidemic- and insulin-sensitizing effects of a ginger (Zingiberofficinale Roscoe) extract in rats fed a high-fat diet. ApplPhysiolNutrMetab 42: 209–215.
- Ghasemi A, Khalifi S, Jedi S (2014) Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. ActaPhysiol Hung 10: 408–20.
- Deeg R, Ziegenhorn J (1983) Kinetic enzymic method for automated determination of total cholesterol in serum. ClinChem 29: 1798–1802.
- Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that proceduces hydrogen peroxide. ClinChem 28: 2077–2080.
- Grove TH (1979) Effect of reagent PH on determination of the high-density lipoprotein cholesterol by precipitation with sodium phototungestate-magnesium. ClinChem 25: 560–564.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of low density lipoprotein cholesterol in plasma without use preparative ultracentri-fuge. ClinChem 18: 499–502.
- Nishikawa SI, Hashi H, Honda K, Fraser S, Yoshida H (2000). Inflammation, a prototype for organogenesis of the lymphopoietic/hematopoietic system. CurrOpinImmunol 12: 342–345.
- Conklin JL (1962). Cytogenesis of the human fetal pancreas, Am J Anat 111: 181–193.
- Laitio M, Lev R, Orlic D (1974) The developing human fetal pancreas: An ultrastructural and histochemical study with special reference to exocrine cells. J Anat 117: 619–634.
- Tadokoro H, Takase M, Nobukawa B (2011) Development and congenital anomalies of the pancreas. Anat Res Int Article ID 351217: 7.
- Inagaki T, Tajiri T, Tate G, Kunimura T, Morohoshi T (2012). Dynamic morphologic change and differentiation from fetal to mature pancreaticacinar cells in rats. J Nippon Med Sch 79: 335–42.
- Caro LG, Palade GE (1964) Protein synthesis, storage, and discharge in the pancreatic exocrine cell: an autoradiographic study. J Cell Biol 20: 473–495.
- Iglesias A, Murga M, Laresgoiti U, Skoudy A, Bernales I et al (2004) Diabetes and exocrine pancreatic insufficiency in E2F1/E2F2 double-mutant mice. J Clin Invest 113: 1398–1407.
- Raeder H, Johansson S, Holm PI et al (2005) Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet 38: 54–62.
- Johansson BB, Torsvik J, Bjørkhaug L et al (2011) Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J BiolChem 286: 34593–34605.
- Kaido M, Fujimura H, Ono A, Toyooka K, Yoshikawa H (1997) Mitochondrial abnormalities in a murine model of primary carnitine deficiency. Systemic pathology and trial of replacement therapy. EurNeurol 38: 302–9.
- Pin CL, Ryan JF, Mehmood R. (2015) Acinar cell reprogramming: a clinically important target in pancreatic disease. Epigenomics 7: 267–81.