Abstract
This study investigated the degradation performance of CuMnO2/C composites synthesized under different conditions in activating PMS. The materials were characterized using techniques such as XRD, SEM, FTIR, XPS, and zeta potential analysis. Additionally, OFX was selected as the target pollutant to examine the influence of catalyst dosage, PMS concentration, and initial pH on the OFX degradation system and to identify the reactive oxygen species involved. Finally, cycling experiments were conducted to evaluate the stability of the composite material and explore the degradation mechanism. The electron spin resonance (ESR) spectroscopy results confirmed that singlet oxygen (¹O₂) was the dominant reactive oxygen species generated during the reaction. X-ray photoelectron spectroscopy (XPS) characterization of the material before and after the reaction revealed that Cu(Ⅰ) served as the active site for activating peroxymonosulfate (PMS) to produce ¹O₂. Additionally, lattice oxygen (Olatt) participated in the redox cycling of metal ions and electron transfer in the CuMnO2/C-PMS system, where Olatt released electrons to facilitate ¹O₂ generation. The incorporation of carbon (C) enhanced the electron transfer capability of Cu species on the catalyst surface, thereby promoting the efficient decomposition of PMS.
Keywords
Carbon materials, Delafossite-type oxides, Advanced oxidation technology, Peroxymonosulfate (PMS), Antibiotics
Introduction
Since the discovery of penicillin in 1929, antibiotics have been widely used in medicine, agricultural production, livestock farming, and other fields. Major classes include tetracyclines (TCs), macrolides (MLs), sulfonamides (SAs), chloramphenicols (CPs), and fluoroquinolones (FQs), significantly improving human health. However, in recent years, the overuse of antibiotics has posed serious threats to both human health and the natural environment. According to statistics, global annual antibiotic consumption ranges from 100,000 to 200,000 tons. The six antibiotics discharged into aquatic environments include ofloxacin (OFX), sulfamethoxazole (SMX), sulfadiazine (SDZ), roxithromycin (ROX), sulfamonomethoxine (SMM), and erythromycin (ERY) [1]. The concentrations of FQs, SAs, and MLs were 121 ng/L, 187 ng/L, and 17.1 ng/L, respectively. SAs were the most dominant antibiotics, accounting for 57.1% of the total antibiotic concentration, followed by FQs at 37.1% [2]. While most antibiotics have relatively short half-lives [3], the continuous release due to overuse and incomplete treatment leads to a substantial annual influx of antibiotics into water environments, resulting in a “pseudo-persistence” phenomenon. Over time, this poses potential risks to human health and ecosystems [4].
Recently, sulfate radical (SO4– )-advanced oxidation processes (SRAOPs) have been widely used for treating organic contaminants in water. SO4– produced from peroxymonosulfate (PMS) possessed a strong oxidation potential, long half-life and high stability in broad pH range (2.0–8.0) [5]. Various methods such as heat or UV treatment [6], carbon-based materials or transition metals activation [7], have been used to improve the PMS catalytic efficiency. Moreover, attentions have been attracted towards transition-metals (Cu–, Co–, Mn–, Fe-based et al.) oxides or their composites construction for PMS activation [8-12]. Wang et al magnetic 2D/2D oxygen-doped graphite carbon nitride/ biochar (γ-Fe2O3/O-g-C3N4/BC) composite was rationally fabricated and used to activate peroxymonosulfate (PMS) for the degradation of SMX,O-g-C3N4 or coconut-derived biochar (BC) displayed low catalytic activity to PMS, while γ-Fe2O3/O-g-C3N4/BC composite showed superior catalytic activity, in which complete degradation of antibiotic sulfamethoxazole (SMX) was quickly achieved, with the mineralization ratio of 62.3%. The surface-bound reactive species (dominant) and sulfate radicals as well as hydroxyl radicals contributed to SMX degradation [13]. Carbon-based materials are commonly used as supports for transition metal catalysts, forming carbon-loaded metal composites. Graphitic carbon nitride (g-C3N4) exhibits unique advantages such as a distinctive electronic structure, stable physicochemical properties, simple preparation methods, and low production costs, making it a promising candidate for advanced oxidation processes (AOPs) in water treatment [14]. Additionally, g-C3N4 consists of heptazine rings with pyridinic nitrogen groups and six lone-pair electrons, enabling it to act as an electron donor [15]. This structure grants g-C3N4 a strong affinity for capturing transition metal ions, thereby enhancing the stability of the prepared samples by reducing the leaching of free metal ions [16]. Previous experimental studies have shown that pure g-C3N4 has limited effectiveness in activating peroxymonosulfate (PMS) and requires further modification to improve its catalytic performance. For instance, researchers have combined g-C3N4 with metal oxides such as Fe3O4, ZnO, and Mn3O4 to develop high-performance catalytic materials with practical applications. Chang et al. prepared catalysts by doping Cu, Co, and Fe into g-C3N4 via calcination to activate PMS for sulfamethoxazole (SMX) degradation, achieving excellent results. The activity of the doped g-C3N4 followed the order: Co > Fe > Cu [17].
To date, there have been no reports on CuMnO2/g-C3N4 composites for PMS activation. Inspired by these findings, we synthesized a carbon-supported metal composite, CuMnO2/C, using g-C3N4 as the carrier, and applied it in peroxymonosulfate activation reactions, and evaluated their catalytic performance on OFX removal in PMS activation system. Three main parts are presented in this study: (i) OFX degradation performance in different Catalysts + PMS systems; (ii) the stability and applications of the CuMnO2/C + PMS system; (iii) the underlying mechanisms of synergistic effects in PMS activation by CuMnO2/C catalyst system through experiments and ESR analysis.
Experimental Section
Chemicals
In this study, most of chemicals are used without further purification, and are purchased from different companies. Cu(NO3)2·3H2O, C4H6MnO4·4H2O, sodium hydroxide, cetyltrimethylammonium bromide (CTAB), ethanol, and melamine were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Potassium peroxymonosulfate (PMS) and ofloxacin (OFX) were obtained from Macklin Biochemical Co., Ltd.
Preparation of Catalyst
CuMnO2
0.15 g of CTAB and 5 mL of 2 M NaOH solution were added to a mixed solution of 25 mL deionized water and 25 mL ethanol. Then, 2.5 mL of Cu(NO₃)₂·3H₂O and C4H6MnO4·4H₂O (both at 0.1 mol/L concentration) were slowly dripped into the mixture. The resulting solution was magnetically stirred for 2 h and then transferred into a 100 mL autoclave for hydrothermal reaction at 160 °C for 22 h. The obtained product was alternately washed with deionized water and ethanol until neutral, dried at 60 °C for 12 h, and finally ground for further use.
g-C3N4
5 g of melamine was weighed using an analytical balance and thoroughly ground in a mortar until it reached a flour-like consistency. The ground powder was then transferred into a 30 mL crucible and placed in a muffle furnace. The temperature was raised to 550 °C at a heating rate of 2.5 °C/min, followed by a 5-hour holding time. After cooling, the calcined product was ground to obtain a yellow powdery material, denoted as g-C₃N₄.
CuMnO2/C
Add 25 mL of deionized water into a beaker, followed by the addition of 37.5 mg of hydrothermally synthesized CuMnO₂ and 0.125 g of calcined g-C₃N₄. Subsequently, introduce 2.5 mL of 3 M NaOH into the mixture. Place the beaker in an ultrasonic bath and sonicate for 90 minutes. After sonication, transfer the solution into a 100 mL autoclave, seal it completely, and react at 160 °C for 22 hours. Once the reaction is complete and the system has cooled, perform vacuum filtration, dry the product, and grind it to obtain the final material.
CuMnO2/g-C3N4
Same as CuMnO₂/C, but without adding NaOH.
Characterization
Powder X-ray diffraction (XRD) with monochromatic Cu Kα90 (λ=1.5406 Å) is recorded by a Bruker AXS D8-Focus diffractometer. The surface morphology is studied using field emission scanning electron microscopy (FESEM,Hitachi SU-8010). X-ray photoelectron spectroscopy (XPS) is examined by the MULTILAB2000 electron spectrometer with 300W Al Kα radiation.
Procedures and Analysis
All degradation Using 10 mg/L ofloxacin (OFX) as the model pollutant, unless otherwise specified, 30 mg catalyst is added into 100 mL 10 mg/L of OFX solution and the suspensions are magnetically stirred for 30 min to obtain adsorption/desorption equilibrium between catalyst and OFX solution, a specified amount of peroxymonosulfate (PMS) was introduced to initiate the degradation reaction. The reaction solution is not buffered and the pH changes during the reaction process are monitored by a pH meter. The pH is adjusted by a diluted aqueous solution of NaOH or HCl. At designated time intervals, 4 mL aliquots of the reaction solution were extracted using a 10 mL syringe, filtered through a 0.22 μm membrane to remove catalyst particles, and the filtrate was analyzed by UV-Vis spectrophotometry to determine the residual antibiotic concentration.
η% =(1-Ct/C0)×100%
where C0 and Ct are the initial and the t min (reaction time) concentration of OFX(mg/L).
Results and Discussion
Characterization of As-prepared Samples
The morphology of the CuMnO₂/C composite was further characterized by SEM. As shown in Figure 1, the loaded material exhibits a transition from an originally smooth surface to a loose, porous structure, while effectively retaining the morphological characteristics of the pristine material [18].

Figure 1: SEM images of CuMnO₂/C
Figure 2 shows the XRD patterns of g-C₃N₄, CuMnO₂, and composite materials synthesized under different conditions. In the pure g-C₃N₄ pattern, two characteristic peaks are observed: a strong peak at 2θ = 27.4° corresponding to the (002) interlayer stacking, and a weaker peak at 2θ = 13.1° attributed to the (100) crystal plane [19.20], reflecting the in-plane ordering of tri-s-triazine structural units. The diffraction peaks of CuMnO₂/g-C₃N₄ and CuMnO₂/C match well with the standard card of monoclinic CuMnO₂ (JCPDS: 50-0860) [21], confirming the successful synthesis of the target phase. Compared to CuMnO₂/g-C₃N₄, the characteristic peaks of g-C₃N₄ in CuMnO₂/C are attenuated, likely due to reduced crystallinity induced by the incorporation of carbon. The structural characteristics of the synthesized catalysts were further investigated through FTIR spectroscopy. As shown in the corresponding figure, pure g-C₃N₄ exhibits: A broad absorption band at 3000-3500 cm⁻¹, attributable to surface-bound H₂O molecules and N-H stretching vibrations [22,23]. A wide absorption range between 1200-1700 cm⁻¹, corresponding to stretching vibrations of aromatic CN-C heterocycles [24]. A characteristic peak at 808 cm⁻¹, representing the breathing mode of s-triazine units [25]. The CuMnO₂/C composite maintains similar characteristic peaks to pristine CuMnO₂. However, the intensity of the 808 cm⁻¹ absorption peak is significantly attenuated in the composite, likely due to: reduction of triazine units in the material and partial substitution of N atoms by C atoms [26].
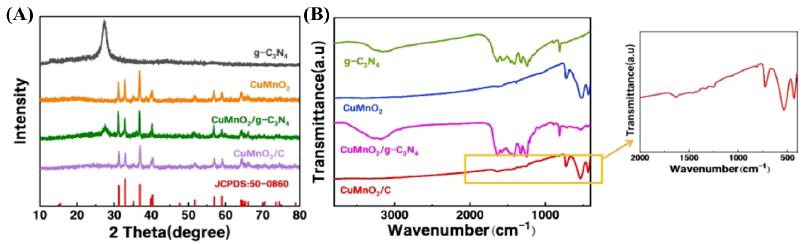
Figure 2: (A) XRD patterns, and (B) FT-IR spectra of as-prepared catalysts.
The variation of surface electrical properties of the CuMnO₂/C catalyst with pH is shown in Figure 3. An isoelectric point exists within the pH range of 3-11. In the pH range of 3-5, CuMnO₂/C exhibits positive surface charge, with the net surface charge reaching zero at pH=5. As pH increases, the zeta potential value progressively decreases and becomes negative, indicating the continuous accumulation of negative charges on the CuMnO₂/C surface, which leads to anion repulsion. The point of zero charge (PZC) of the catalyst significantly influences its adsorption and catalytic properties.

Figure 3: Zeta potential images of CuMnO₂/C
Catalytic Performance of Different Catalysts for OFX Degradation
Through preliminary comparative experiments and synthesis condition optimization, the optimal composite material was determined. Using OFX as the target pollutant at a concentration of 10 mg/L, the catalyst was added and allowed to adsorb for 30 minutes before PMS was introduced for a 120 minute reaction. As shown in Figure 4: Pure g-C₃N₄ showed negligible degradation effects on OFX, CuMnO₂/g-C₃N₄ and CuMnO₂/C achieved OFX removal rates of 76% and 83%, respectively. The physical mixture of CuMnO₂ and g-C₃N₄ demonstrated a 67% removal rate. The results clearly indicate that CuMnO₂/C exhibits the best OFX degradation performance, which aligns with the aforementioned characterization data. This composite material therefore warrants further investigation.
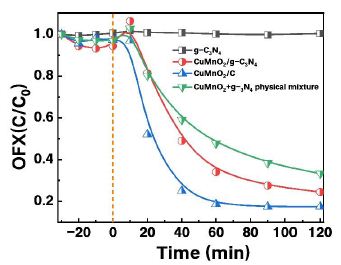
Figure 4: The degradation efficiency in different PMS/catalysts system. Reaction conditions: [catalyst] =0.3 g/L, [PMS] = 0.33 mM, [OFX] = 10 mg/L, initial pH =6.5, T = 30 ℃.
Different Influence Factors
To demonstrate the optimal performance of the CuMnO2/C composite material, further studies were conducted to investigate the effects of PMS concentration, catalyst dosage, and initial pH of the solution on the degradation of OFX. First, the degradation effect of OFX was studied by varying the concentration of PMS. The selected PMS concentrations were 0.24, 0.33, and 0.49 mM. As shown in Figure5A, as the PMS concentration increased from 0.24 mM to 0.33 mM, the removal rate of OFX increased from 83% to 88%. This is attributed to the increasing number of PMS molecules adsorbed on the surface of the CuMnO2/C composite material, which generates more reactive oxygen species (ROS). However, when the PMS concentration was increased to 0.49 mM, the removal rate of OFX did not increase further. This is because an excess of PMS can react with ROS, thereby inhibiting the degradation of OFX.
Next, we selected catalyst concentrations of 0.2 g/L, 0.3 g/L, and 0.4 g/L as variables to study the effect of catalyst content on the degradation of OFX in the activation system. As shown in Figure 5B, the degradation of OFX by CuMnO2/C activated PMS at different catalyst concentrations is illustrated. As the catalyst concentration increased from 0.2 g/L to 0.3 g/L, the degradation rate of OFX rose from 81% to 88%. However, when the catalyst concentration reached 0.4 g/L, the degradation effect showed an inflection point, and the removal rate of OFX did not increase further, remaining at 87%. With the continuous increase in catalyst concentration, the surface active sites became saturated and could no longer provide additional sites for activating PMS to generate more reactive oxygen species. Therefore, in the CuMnO2/C activated PMS system for degrading OFX, the optimal catalyst concentration is 0.3 g/L.
The pH of the solution has a significant impact on the organic compounds and the existing forms of PMS [27]. We investigated the effect of different pH levels (3.47-9.6) on the removal of OFX using CuMnO2/C activated PMS. Figure 5C illustrates the degradation of OFX at different initial pH values. As the pH increased from 3.47 to 6.5, the removal rate of OFX gradually increased. However, when the pH reached 9.6, the removal rate of OFX slightly decreased. This is because an excess of OH⁻ accumulating on the surface of the catalyst can lead to stronger electrostatic repulsion between the PMS anions, which reduces the availability of SO•⁻ 4 and subsequently affects the degradation process. Overall, the CuMnO2/C activated PMS system for degrading OFX demonstrates a wide pH adaptability range.
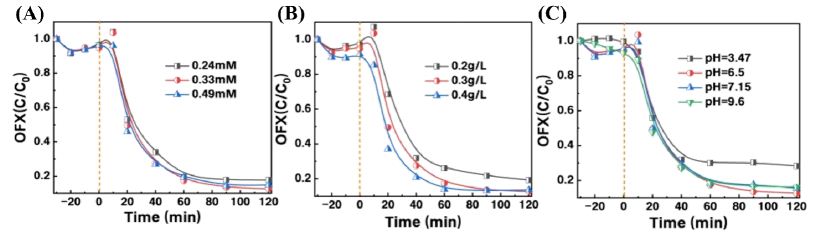
Figure 5: Effect of experimental conditions on OFXremoval in CuMnO2/C + PMS system. (A) PMS dosage (0.24 mM–0.49 mM); (B) catalyst dosage (0.2 g/L–0.4 g/L); (C) initial pH conditions. Reaction conditions: T = 30 ℃.
Universality and Recyclability of CuMnO2/C
Furthermore, the long-term performance of CuMnO2/C was evaluated. Under the conditions of a catalyst dosage of 0.03 g and a PMS dosage of 0.33 mM, as shown in Figure 6A, after four consecutive cycles, the removal efficiency of OFX by CuMnO2/C-activated PMS experienced a certain degree of decline—potentially due to the leaching of some metal active components—but still reached 76%, indicating the relatively good stability of CuMnO2/C. Figure 6B displays the XRD pattern of CuMnO2/C after the PMS-activated degradation of OFX. Compared with the XRD pattern before the reaction, the structure of CuMnO2/C showed no significant changes, confirming that the crystalline structure of the catalyst remained stable after the OFX degradation reaction.
As shown in Figure 6C, in real water environments (Lanyue Lake water and tap water), the CuMnO2/C-activated PMS system still efficiently degraded OFX. In the tap water system, the degradation efficiency after 120 minutes of reaction was nearly identical to that in the deionized water system (~88%), indicating that the complex composition of tap water did not negatively affect OFX removal. This may be attributed to various inorganic ions and other substances in the water promoting the reaction through mechanisms such as chelation, adsorption bridging, and the generation of reactive species via radical reactions. In the Lanyue Lake water system, the OFX degradation rate reached 84% after 120 minutes, slightly lower than in the deionized water system but still demonstrating excellent degradation performance.
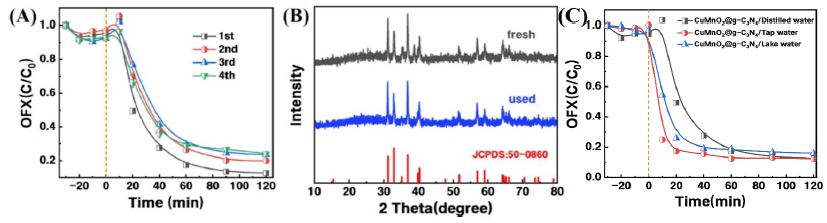
Figure 6: Universality and recyclability study on OFX removal in CuMnO2/C + PMS system. (A) The reusability of CuMnO2/C; (B) fresh and used CuMnO2/C characterization XRD; (C) different water qualities. Reaction conditions: [OFX] = 0.01 g/L, [catalyst] = 0.3 g/L, [PMS] = 0.33 mM, pH =6.5, T = 30 ℃.
Identification of the Main Active Species
Figure 7A shows the results of radical scavengers on the degradation of OFX in the CuMnO2/C activated PMS system. It can be observed that the addition of 0.6 mM TBA and 0.6 M MeOH removed 73% and 80% of OFX, respectively, indicating the presence of a small amount of •OH in the system [28]. To further confirm these results, ESR tests were conducted. A 1: 1: 1 triplet signal characteristic of TEMP-1O2 was detected 2 minutes after the addition of the catalyst [29]. As the reaction progressed to the 20th minute, the TEMP-1O2 signal remained unchanged, while the intensity of the TEMP-1O2 signal significantly increased. The combined results of the quenching experiments indicate that in the CuMnO2/C catalytic degradation of OFX system, 1O2 plays a major role in the degradation of the pollutant.
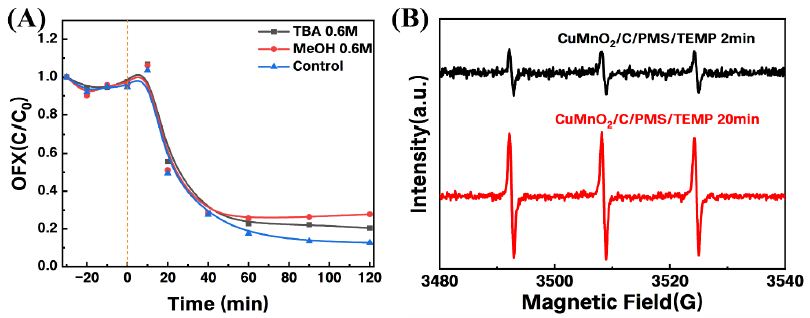
Figure 7: Effects of radical scavengers on OFX degradation; (B) ESR spectrum of TEMP for 1O2 in CuMnO2/C + PMS system.
The Possible Mechanisms of PMS Activation Over CuMnO2/C
To investigate the changes in elemental content of the CuMnO2/C catalyst before and after the reaction and to identify the active sites, as well as to clarify the degradation mechanism, XPS was employed to analyze the elemental composition and valence states of the catalyst, along with peak fitting analysis. As shown in Figure 8 (A) displays the C1s spectrum, where the peak of C1s significantly enhanced after the reaction. This may be due to the surface adsorption of a certain amount of target pollutants during the reaction, which corresponds to the change in C1s observed in Figure 8A. In Figure 8B, the O1s spectrum is divided into three peaks at 529.58 eV, 531.27 eV, and 533.0 eV, corresponding to lattice oxygen (Olatt), surface hydroxyl oxygen (Oads), and adsorbed oxygen from water, respectively. The relative content of Olatt significantly decreased from 66.3% to 11% after the catalytic reaction, while the content of Oads increased from 29% to 41%. This indicates that both Olatt and Oads participated in the redox reactions and electron transfer of metal ions in the CuMnO2/C activated PMS system, with lattice oxygen being able to release electrons to generate 1O2. Additionally, the increase in the proportion of H2O is attributed to the participation of CuMnO2/C in the aqueous phase reaction, resulting in the adsorption of a significant amount of crystalline water.
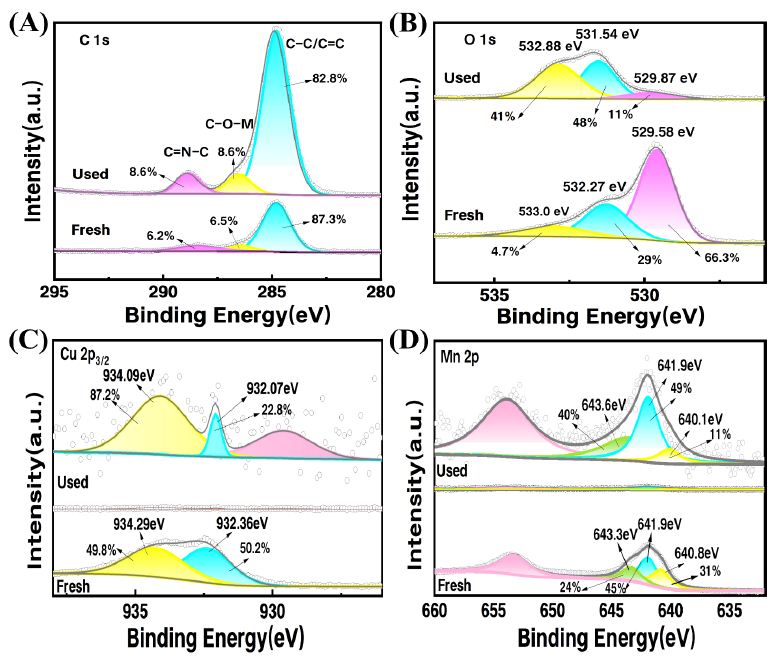
Figure 8: XPS spectra of (A) C 1 s; (B) O 1 s; (C) Cu 2p; (d)Mn 2p for CuMnO2/C before and after reaction.
Figures 8C and 8D present the high-resolution XPS spectra of Cu2p3/2 and Mn2p3/2. Compared to before the reaction, the peaks for Cu and Mn elements after the reaction became less pronounced, which aligns with the significant reduction in the Cu2p and Mn2p peak intensities in the full spectrum after the reaction. In Cu2p3/2, the binding energies of 932.36 eV and 934.29 eV correspond to Cu(I) and Cu(II) [30,31], respectively. After the reaction, the relative content of Cu(I) decreased from 50.2% to 22.8%, while the relative content of Cu(II) increased from 49.8% to 87.2%. This indicates that Cu(I) can provide electrons during the catalytic process, leading to an increase in Cu(II) after oxidation, and Cu(II) can also accept electrons during the reaction process to form Cu(I). In Figure 8D, the Mn2p3/2 peak is divided into three peaks at 640.8 eV, 641.9 eV, and 643.3 eV, corresponding to Mn(II), Mn(III), and Mn(IV), respectively [32,33]. After the reaction, Mn remained in the states of Mn(II), Mn(III), and Mn(IV). The XPS results before and after the reaction indicate that Cu(I) acts as the active site in the system, activating peroxymonosulfate to produce reactive oxygen species (1O2). Additionally, the incorporation of C enhances the electron transfer capability of Cu species on the catalyst surface, thereby promoting the effective decomposition of PMS (Figure 9).
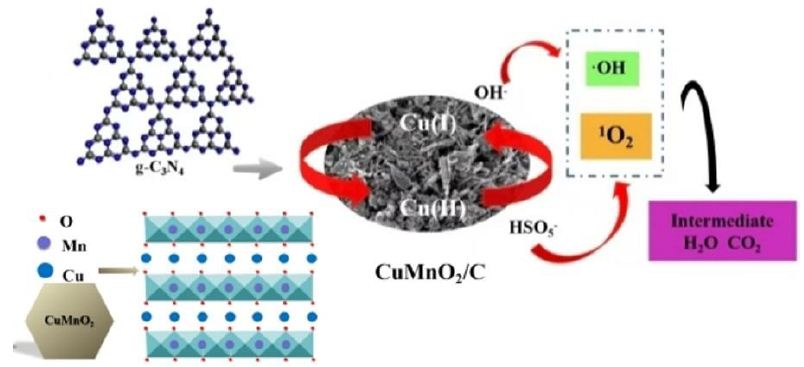
Figure 9: Mechanism of PMS activation on CuMnO2/C for OFX degradation.
Conclusions
In summary, this study successfully prepared the CuMnO2/C composite material via a hydrothermal method. The optimal conditions were determined to be a PMS concentration of 0.33 mM, catalyst dosage of 0.3 g/L, and neutral pH, achieving an 88% removal rate of OFX after 120 minutes of reaction. ESR analysis confirmed that singlet oxygen (1O2) served as the primary reactive species. In this system, Cu(Ⅰ) acted as the active site for peroxymonosulfate activation, generating reactive oxygen species (1O2). Simultaneously, lattice oxygen (Olatt) participated in the redox cycling of metal ions and electron transfer within the CuMnO2/C-PMS system, where the release of electrons from lattice oxygen contributed to 1O2 production. The incorporation of carbon enhanced the electron transfer capability of surface Cu species, thereby promoting the efficient decomposition of PMS. The applicability of the CuMnO2/C + PMS system in a wide pH range of 3.47~9.6 and various organic pollutants and different water qualities validates its application potentials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting Information
Supplementary data associated with this article can be found in the Supporting Information.
References
- Kümmerer K (2009) Antibiotics in the aquatic environment–A review–Part I [J]. Chemosphere 75: 417-434. [crossref]
- Lu P, Fang Y, Barvor J B, Neth N L K, Fan N, et al. (2019) Review of antibiotic pollution in the seven watersheds in China [J]. Polish Journal of Environmental Studies 28: 4045-4055.
- Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance [J]. Environmental Science & Technology 49: 6772-6782. [crossref]
- Zeng H, Li J, Zhao W, Xu J, Xu H, et al. (2022) The current status and prevention of antibiotic pollution in groundwater in China [J]. International Journal of Environmental Research and Public Health 19: 11256. [crossref]
- Guo Y, Zeng Z, Zhu Y, Huang Z, Cui Y, et al. (2018) Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene [J]. Applied Catalysis B: Environmental 220: 635-644.
- Rehman F, Sayed M, Khan JA, Shah NS, Khan HM, et al. (2018) Oxidative removal of brilliant green by UV/S2O82‒, UV/HSO5‒and UV/H2O2 processes in aqueous media: a comparative study [J]. Journal of Hazardous Materials 357: 506-514. [crossref]
- Wang J, Cai J, Wang S, Zhou X, Ding X, et al. (2022) Biochar-based activation of peroxide: multivariate-controlled performance, modulatory surface reactive sites and tunable oxidative species [J]. Chemical Engineering Journal.428: 131233.
- Liang H, Sun H, Patel A, Shukla P, Zhu Z, et al. (2012) Excellent performance of mesoporous Co3O4/MnO2 nanoparticles in heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions [J]. Applied Catalysis B: Environmental 127: 330-335.
- Pang Y, Lei H (2016) Degradation of p-nitrophenol through microwave-assisted heterogeneous activation of peroxymonosulfate by manganese ferrite [J]. Chemical Engineering Journal 287: 585-592.
- Qiu Y, Zhang Q, Wang Z, Gao B, Fan Z, et al. (2021) Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study [J]. Science of the Total Environment 758: 143584. [crossref]
- Saputra E, Muhammad S, Sun H, Ang H-M, Tadé M O, et al. (2013) Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions [J]. Applied Catalysis B: Environmental.142: 729-735.
- Liang F, Liu Z, Jiang X, Li J, Xiao K, et al. (2023) NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal [J]. Chemical Engineering Journal 454: 139949.
- Wang S, Wang J (2022) Magnetic 2D/2D oxygen doped g-C3N4/biochar composite to activate peroxymonosulfate for degradation of emerging organic pollutants [J]. Journal of Hazardous Materials 423: 127207. [crossref]
- Haque E, Jun JW, Talapaneni SN, Vinu A, Jhung SH (2010) Superior adsorption capacity of mesoporous carbon nitride with basic CN framework for phenol [J]. Journal of Materials Chemistry 20: 10801-10803.
- Sun B, Ma W, Wang N, Xu P, Zhang L, et al. (2019) Polyaniline: a new metal-free catalyst for peroxymonosulfate activation with highly efficient and durable removal of organic pollutants [J]. Environmental Science & Technology 53: 9771-9780. [crossref]
- Sukeshini AM, Kobayashi H, Tabuchi M, Kageyama H (2000) Physicochemical characterization of CuFeO2 and lithium intercalation [J]. Solid State Ionics 128: 33-41.
- [Oh W-D, Chang VW, Hu Z-T, Goei R, Lim T-T (2017) Enhancing the catalytic activity of g-C3N4 through Me doping (Me= Cu, Co and Fe) for selective sulfathiazole degradation via redox-based advanced oxidation process [J]. Chemical Engineering Journal 323: 260-269.
- Liu M, Zheng L, Bao X, Wang Z, Wang P, et al. (2021) Substrate-dependent ALD of Cux on TiO2 and its performance in photocatalytic CO2 reduction [J]. Chemical Engineering Journal 405: 126654.
- Guo F, Wang L, Sun H, Li M, Shi W (2020) High-efficiency photocatalytic water splitting by a N-doped porous g-C3N4 nanosheet polymer photocatalyst derived from urea and N, N-dimethylformamide [J]. Inorganic Chemistry Frontiers 7: 1770-1779.
- Li F, Xu B, You X, Gao G, Xu R, et al. (2023) In-situ synthesis of Pd nanocrystals with exposed surface-active facets on g-C3N4 for photocatalytic hydrogen generation [J]. International Journal of Hydrogen Energy 2023 48: 12299-12308.
- Dai C, Tian X, Nie Y, Fu W, Wang J (2023) Effect of the interaction mode of H2O2 over CuMnO2 surface on• OH generation for efficient degradation of ofloxacin: Activity and mechanism [J]. Chemical Engineering Journal 451: 138749.
- Zhang X, Xie X, Wang H, Zhang J, Pan B, et al. (2013) Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging [J]. Journal of the American Chemical Society 135: 18-21. [crossref]
- Wang Y, Wang X, Antonietti M (2012) Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry [J]. Angewandte Chemie International Edition 51: 68-89. [crossref]
- Chu S, Wang Y, Guo Y, Feng J, Wang C, et al. (2013) Band structure engineering of carbon nitride: in search of a polymer photocatalyst with high photooxidation property [J]. Acs Catalysis 3: 912-919.
- Liu J, Zhang T, Wang Z, Dawson G, Chen W (2011) Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity [J]. Journal of Materials Chemistry 21: 14398-14401.
- Li H, Zhou Y, Tu W, Ye J, Zou Z (2015) State‐of‐the‐art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance [J]. Advanced Functional Materials 25: 998-1013.
- Wang C, Yang Q, Li Z, Lin K-Y A, Tong S (2019) A novel carbon-coated Fe-C/N composite as a highly active heterogeneous catalyst for the degradation of Acid Red 73 by persulfate [J]. Separation and Purification Technology 213: 447-455.
- Tian X, Luo T, Nie Y, Shi J, Tian Y, et al. (2022) New insight into a Fenton-like reaction mechanism over sulfidated β-FeOOH: key role of sulfidation in efficient iron (III) reduction and sulfate radical generation [J]. Environmental Science & Technology 56: 5542-5551. [crossref]
- Wu F, Nie X, Nie Y, Dai C, Tian X (2023) Layered double hydroxide driven 1O2 non-radical or• OH radical process for the degradation, transformation and even mineralization of sulfamethoxazole via efficient peroxymonosulfate activation [J]. Separation and Purification Technology 318: 123969.
- Gusain R, Kumar P, Sharma O P, Jain S L, Khatri O P (2016) Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation [J]. Applied Catalysis B: Environmental 181: 352-362.
- Yin C, Zhou S, Zhang K, Bai J, Lv Y, et al. (2021) Crednerite CuMnO2 as highly efficient Fenton-like catalysts for p-nitrophenol removal: Synergism between Cu (I) and Mn (III) [J]. Journal of Cleaner Production 319: 128640.
- Dong N, Chen M, Ye Q, Zhang D, Dai H (2022) An investigation on catalytic performance and reaction mechanisms of Fe/OMS-2 for the oxidation of carbon monoxide, ethyl acetate, and toluene [J]. Journal of Environmental Sciences 112: 258-268. [crossref]
- Biesinger MC, Payne BP, Grosvenor AP, Lau LW, Gerson AR, et al. (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni [J]. Applied Surface Science 257: 2717-2730.