Abstract
Leukaemia inhibitory factor (LIF), a cytokine in the interleukin 6 family, is considered a pleiotropic molecule with diverse functions and is expressed in different tissues and cell types. The role of LIF in the reproductive system during the implantation process has been described; however, to date, there is little available information about the effect of LIF on the function and development of the female gonad. The focus of this review is to analyse the structure of LIF, the signalling pathway involved, and the expression of LIF and its receptor in different ovarian cell types. In addition, the participation of LIF and its receptor in ovarian function, follicular development, steroidogenesis and ovulation is discussed.
Introduction
Leukaemia inhibitory factor (LIF) is a cytokine that belongs to the interleukin 6 (IL-6) family and is considered a pleiotropic molecule since it is expressed in different tissues and cell types and has diverse functions. The first observed effect of LIF was its ability to function as a differentiation inducer and proliferation inhibitor of the myeloid leukemic cell line (M1) to macrophages in an in vitro model [18]. Other specific functions of LIF have been reported, such as its participation in bone resorption [36,37] neonatal neuronal transdifferentiation [40,41] and its involvement in cardiac remodelling [19-21], folliculogenesis and spermatogenesis [14]
Currently, the study of this cytokine in the female reproductive system has attracted interest since it is found in different tissues of the female reproductive system [22]. LIF was initially discovered to be necessary for the uterine implantation process [10], and in recent decades, it has been found to be present in the oviduct [23] and ovary, but its functions at these levels have not been fully understood. In the ovaries of different species and from different study models, both in vitro and in vivo, LIF has been shown to fulfil important functions depending on folliculogenesis stage, mainly during the neonatal and fertile periods of female reproductive life; however, the role of LIF during the female subfertile period is unclear. Therefore, in the present review, we discuss the available data on the role of LIF in ovarian folliculogenesis during female reproductive life.
Lif and Its Receptor
LIF is a protein with an approximate molecular weight of 20 kDa, but its molecular weight can range from 38-67 kDa due to differences in posttranslational modifications [38,39]. Among the modifications that mature proteins present are glycosylations, which are mainly associated with asparagine residues. Although glycosylations explain, to some extent, the variations in the molecular weight of LIF (38-67 kDa) [3], we still cannot determine how the glycosylation pattern affects the function and stability of the protein. LIF is described as long-chain cytokine with four α-helices in an up-down-up configuration, as has also been shown for other IL-6 family members such as ciliary neurotrophic factor (CNTF), growth hormone (GH), granulocyte colony-stimulating factor [42,45] Although a low degree of homology is observed between the primary structures of these cytokines, they show a high degree of homology in their tertiary structures and in the functional epitopes of their receptors, as demonstrated by X-ray crystallography resonance imaging [43].
Lif Receptors as a Heterodimer and Associated Signalling Pathways
For LIF to carry out its action, it must interact with a heterodimeric plasmatic membrane receptor formed by two proteins, gp130 and LIFRβ. The LIFRβ subunit which can also interact with other cytokines of the IL-6 family, such as CNTF and oncostatin M and the gp130 is a subunit common for all IL-6 family cytokines [46]. LIF interacts specifically and directly with the LIFRβ subunit but with a relatively low affinity (Kd=1∗10−9). When the gp130 subunit interacts with the LIF-LIFRβ complex, a high-affinity trimeric LIF-LIFRβ-gp130 complex is formed (Kd=1∗10^−10), which is necessary for receptor activation and therefore intracellular signalling [24]. The interaction between LIF and LIFRβ is 80-fold greater than that between LIF and gp130, which is not surprising given that gp130 also interacts with other cytokines [25].
IL-6 family cytokine-associated receptors do not exhibit kinase activity. The binding of LIF to its heteroreceptor causes conformational changes in the subunits that allow cytoplasmic activation of Janus kinase (JAK), tyrosine phosphorylation of the heteroreceptor and phosphorylation of signal transducer and activator of transcription (STAT). It has been observed that LIF can activate the JAK1/STAT3 pathway, which is considered to be the canonical signalling pathway involved (Figure 1), but importantly, the JAK/STAT signalling cascade is a signalling pathway shared by several cytokine receptors [47]. In addition to activating the JAK/STAT pathway, the interaction between LIF and LIFR can activate other signalling pathways, such as the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways; however, the effects vary and may even be opposite depending on the cell type involved, having been observed to either induce or inhibit cell differentiation in a variety of cases [48].
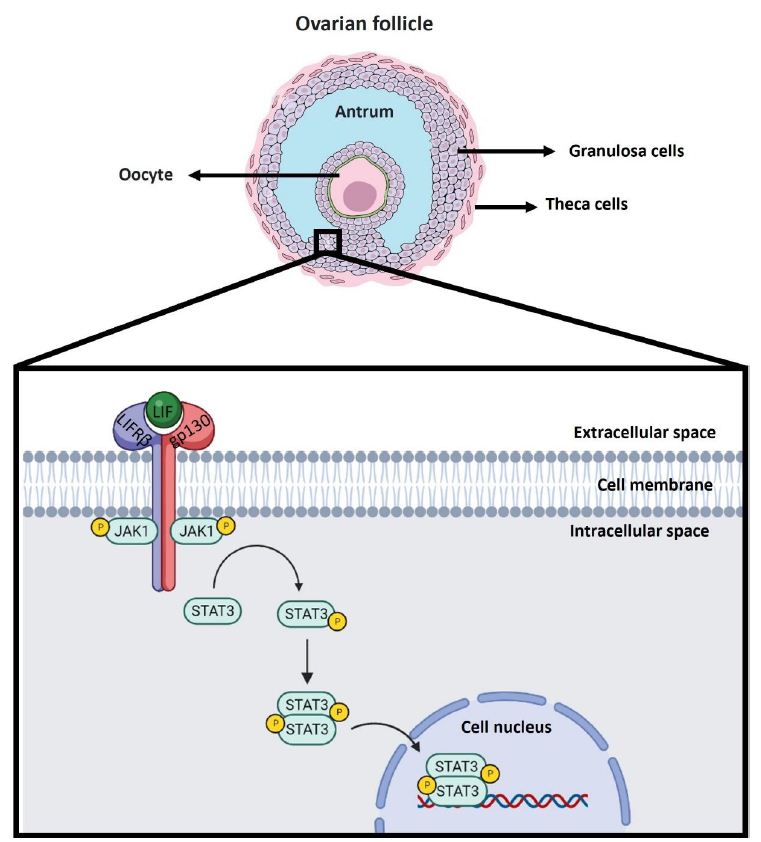
Figure 1: Canonical LIF signaling pathway in the ovarian follicle. LIF is expressed in the ovaries of various animal models, such as mice, rats, nonhuman primates, and humans. Specifically, it has been observed that LIF is expressed in different ovarian cells such as theca cells, granulosa cells and oocyte. In the scheme, granulosa cells of antral follicles are used as an example of localization of the signaling cascade associated with LIF. The LIF molecule is shown in green. The LIF receptor as a heterodimer consists of the LIFRβ subunit in blue and the gp130 subunit in red. Upon interaction of LIF with the LIFRβ subunit of the LIF receptor, the gp130 subunit is recruited to form the heterotrimer. When this occurs the signaling cascade is triggered where JAK1 phosphorylation is induced to be activated. JAK1 upon activation subsequently phosphorylates STAT3 so that it can homodimerize and translocate to the nucleus to act as a transcription factor in the regulation of gene expression.
Role of Lif in the Female Reproductive System
Function of Lif In Utero
It has been widely reported that LIF participates in the implantation process in the uterus of several mammals [50,51]. An increase in LIF levels in utero has been observed at 2 crucial moments of pregnancy: the first is in the oestrus stage, coinciding with the ovulation process [57], and the second is on the 4th and 5th days of pregnancy. In situ hybridization has shown that LIF expression in pregnant mice is confined to the endometrial glands [39]. After the 5th day of pregnancy, once implantation and decidualization occur, the glands begin to degenerate, and LIF secretion ceases. This observation suggests that the peak LIF signal produced during pregnancy could be decisive at the time of implantation; this was corroborated in female LIF-deficient (LIF-/-) homozygous mice whose ova fertilized by either LIF-/- or wild-type (WT) males reached the blastocyst stage without problems. However, these mice could not reach the implantation stage [49] when these embryos were transferred to a pseudopregnant WT female, the pregnancy reached full term, suggesting that the implantation failure was due to a maternal defect, which was essentially attributed to the lack of LIF. Based on these studies, LIF was found to be a fundamental factor in the implantation process. However, the action of LIF in this process is subject to regulation by other factors, including kisspeptin (KP).
KP is a key neuropeptide involved in the regulation of reproductive function through the hypothalamic-pituitary-gonadal (HPG) axis [52]. Despite the important role of the KP at the central level, it also regulates gonadotrophins secretion [26,27]. Calder et al. confirmed that KP (Kiss1-/-) deficient female mice mated with WT males are unable to achieve pregnancy due to implantation defects [11]. This finding led us to consider whether Kiss1-/- females lacked some determinant signals for the implantation process. In this regard, as mentioned above, studies have shown that LIF is an essential factor for the implantation process in mice. Indeed, a therapy based on the exogenous administration of recombinant LIF was able to partially rescue the implantation process in Kiss1-/- females. Based on these findings, it was hypothesized that LIF expression was reduced in female Kiss1-/- mice, which was subsequently confirmed by the marked reduction in LIF expression at the level of the uterine glandular lumen in Kiss1-/- females compared to WT female mice. This is the first study to demonstrate that uterine KP signalling regulates glandular levels of LIF.
Function of Lif in Ovary
LIF is expressed not only in endometrial tissue in the female reproductive system but also in the ovary (Senturk & Arici 1998). LIF is expressed in the ovaries of various animal models, such as mice, rats, nonhuman primates, and humans [5,6,31,32]. During the fertile stage in human and nonhuman primates, LIF has been shown to be present in the follicular fluid of preovulatory follicles [30]. In rats, ovarian LIF levels change during the oestrous cycle, with the highest levels being observed at the night of proestrus, corresponding to oestrus and metaestrus/diestrous [28,29]. We have seen in a rat model that the ovarian expression of LIF, in addition to being different during the oestrous cycle, is different during the reproductive life of the rat, as indicated by a greater expression of this cytokine in the fertile stage and a markedly lower expression during lactation. In neonatal rats, LIF is localized to granulosa cells in primordial and primary follicles and in oocytes [53]. In mouse ovaries, LIF is localized to cumulus cells and oocytes from antral follicles. In these cells, the intensity of the LIF marker increases in growing and mature follicles [54]. According to the IHC technique, LIF is localized in theca cells, granulosa cells and oocytes from healthy antral follicles and mainly in luteal cells of the corpus luteum in fertile rats in the oestrous stage [58]. These data suggest a possible autocrine/paracrine role of LIF during the neonatal and fertile periods in females, as well as a role in the stages of cyclical recruitment, ovulation, corpora lutea development and steroid hormone production.
This paracrine or autocrine action of LIF is suggested by the fact that ovarian cells express LIF receptors. The LIF receptor has been found to localize to different ovarian follicular cells in different species [44]. In the ovaries of fertile female monkeys, LIFRβ and gp130 (also known as IL6ST) are localized by IHQ in theca cells and granulosa cells of antral follicles [33]. In humans, LIFRβ and gp130 have been identified by RT‒qPCR and IHQ in granulosa cells and oocytes from primordial follicles of foetal ovaries and in granulosa cells from primary and secondary follicles in adult ovaries [1]. The activation of the LIF receptor in ovarian follicular cells of monkeys in the fertile stage and in the human granulosa cell line COV434 is related to the signalling pathway corresponding to JAK1/STAT3 (Figure 1) and phosphorylated STAT3 after an ovulatory stimulus [35]. On the other hand, in vitro studies in pig ovaries revealed an increase in the phosphorylated form of STAT3 associated with the cumulus–oocyte complex [34]. We recently reported that, in rat ovaries incubated with LIF for 30 minutes, STAT3 phosphorylation increases [37]. LIF and its receptors can activate other signalling pathways [23] in a paracrine or autocrine manner. It must be determined whether these signalling pathways can also be activated in the ovary, for which further studies are needed.
Participation of LIF in Follicular Recruitment
LIF has been shown to promote the transition from primordial to preantral follicle in a neonatal rat and goat in vitro study [58,59] and it has been proposed that this effect in rats is indirect and mediated by an increase in the expression of kit ligand (KL), a known factor that promotes the passage from primordial to primary follicles. We recently published results that support the idea that chronic treatment with LIF for 28 days in vivo decreases the total number of small primary and secondary follicles in the ovaries of fertile rats. In contrast, the number of primordial follicles does not change with LIF treatment and therefore does not explain the decrease found in primary and secondary follicles. LIF has been shown to decrease the growth of developing follicles both in vitro in prepubertal mice [55] and in vivo in fertile rats [56] Specifically, it has been observed in vitro that both secondary and antral follicles are small in size when ovaries are incubated with LIF [60]. This decrease in the development of preantral follicles disagrees with the results of Nilsson et al. and may be due to the chronic in vivo treatment of LIF. However, the effects of LIF on apoptosis are still controversial [16]. To determine whether LIF induces apoptosis in vivo, it is necessary to carry out studies with shorter treatment durations because at 28 days, no pyknotic nuclei were observed during the morphology analysis. LIF regulates the recruitment of primordial follicles, which is relevant for maintaining the cohort of reserve follicles in the ovary. Its effect could be associated with the maintenance and avoidance of a massive loss of the ovarian follicular reserve during reproductive life. Studies focused on the subfertility stage are also necessary.
Follicular atresia corresponds to the degeneration or death of the ovarian follicle so that healthy follicles can develop normally, while defective follicles degenerate and die by apoptosis or autophagy, depending on the stage of follicular development [12,51]. Autophagic atresia has been documented to occur mainly in preantral follicles, whereas apoptosis-induced atresia occurs mainly at the antral follicle stage during cyclical recruitment. It has been observed that atresia in antral follicles is due to the lack of FSH signalling [53] and that this process is associated with the activation of the LIF-STAT3 pathway in the granulosa cells of bovine ovarian follicles [24]. FSH is important for the selection and development of antral follicles, mainly through cyclical recruitment [66]. However, treatment with LIF in rat ovaries for 28 days does not induce follicular atresia, and serum FSH levels do not change with respect to those of the control at the end of treatment. These results suggest that LIF alone does not have an effect on follicular atresia, and it is probable that atresia due to lack of FSH is due to another mechanism and not through LIF.
Participation of LIF in Ovulation and Corpus Lutea
The effect of LIF on ovulation has been evaluated by Murphy et al., 2016. In this work, LIF concentrations were determined in the follicular fluid of preovulatory follicles of fertile female rhesus macaques, and an increase in LIF was observed after hCG administration as an ovulatory stimulus and prior to ovulation. A similar phenomenon has been observed in follicular fluid from preovulatory follicles in humans after hCG has been administered [13]. These results support the premise that LIF is produced in granulosa cells, cumulus cells, and oocytes, as has been observed in rodents [65]. LIF is produced during all stages of follicular development, apparently regulating the growth and maturation of follicles and oocytes and ultimately contributing to ovulation. In summary, the data suggest that LH can stimulate the production and secretion of LIF in granulosa cells, specifically in preovulatory follicles, which express the LH receptor (LHR), to promote ovulation. For example, the administration of hCG (500 IU/ml) provoked a significant increase in intrauterine LIF, VEGF and MMP-9 (Licht et al., 2007).
There are no data in humans that evaluate the effect of LIF on ovulation itself, but when determining the concentration of LIF in the follicular fluid and in the serum of women suffering from polycystic ovarian syndrome (PCOS), a condition characterized by oligo- or anovulation, women with PCOS have decreased levels of LIF compared to what is observed in control women [64]. A study carried out in a rat model revealed that local LIF administration to the ovary for 28 days can increase the number of large corpora lutea and the serum progesterone concentration at the end of 28 days of treatment with LIF. Large corpora lutea are associated with recent ovulation of preovulatory follicles and increased progesterone production [15]. This is because the luteal cells of newly formed corpora lutea express higher levels of 3β-hydroxysteroid dehydrogenase (3β-HSD) than do those of involuting corpora lutea (from previous ovulations), suggesting that LIF could be important for the ovulatory process. In addition, luteal cells present positive immunoreactivity for LIF in the ovaries of fertile rats, and the highest levels of the messenger RNA that codes for LIF are detected in oestrous and metaestrus/diestrus [63], stages of the oestrous cycle, where the greatest amount of progesterone is produced and newly formed corpora lutea are observed. It is possible that LIF may also influence the survival of the corpora lutea, but this possibility requires further study. LIF not only locally regulates the ovulatory process but also participates at the central nervous system level. LIF induces an increase in GnRH at the hypothalamic level, regulating reproductive function locally in the gonad and in the central nervous system [17].
LIF Involvement in Ovarian Steroidogenesis
However, studies regarding the effect of LIF on steroidogenesis are rare. The first observations of the possible effect of LIF on this process were obtained from experiments carried out in the adrenal cortex and in the human adrenocortical cell line NCI-H295R, where the results indicated that LIF could increase the secretion of cortisol and aldosterone through a mechanism mediated by ACTH [4,8]. On the basis of these findings, LIF can increase the expression of the regulatory protein of acute steroidogenesis (StAR) [62], and in vitro studies of Leydig cells from immature rats incubated with different concentrations of LIF revealed that at low concentrations, this cytokine could increase androgen production, apparently increasing the expression of StAR and 17-hydroxysteroid dehydrogenase 3 (Hsd17b3) [61]. In both cases, LIF can increase the expression of the StAR protein in vitro, which suggests, on the one hand, that the increase in steroid hormone levels in vitro is due to this effect and, on the other hand, that this phenomenon could be replicated in cells of other steroidogenic tissues, such as the ovary. However, until now, there has been no published evidence supporting the involvement of LIF in enzymes or transporters involved in ovarian steroidogenesis.
Conclusions and Perspectives
LIF is a pleiotropic cytokine that has various functions and activates various intracellular signalling pathways, depending on the cell type and tissue in which it participates. The LIF-LIFR system has been studied in the immune system and cancer, and its therapeutic role has been studied in various pathologies; its participation in the implantation process and therapeutic use in the reproductive system have been described. Recently, publications on the role of the male gonad in development and spermatogenesis have emerged. In this review, we analysed the participation of LIF in the ovary and discussed its possible signalling pathways and localization in different cell types in the female gonad. LIF is expressed at different levels during the oestrous cycle stage, and during ovary development, it participates in follicular development, ovulation (Figure 2) and steroidogenesis. We cannot exclude our analysis because, in the context of infertility pathology caused by ovarian dysfunction, LIF could also play a key role in considering future therapy or therapeutic use, but further studies are needed.
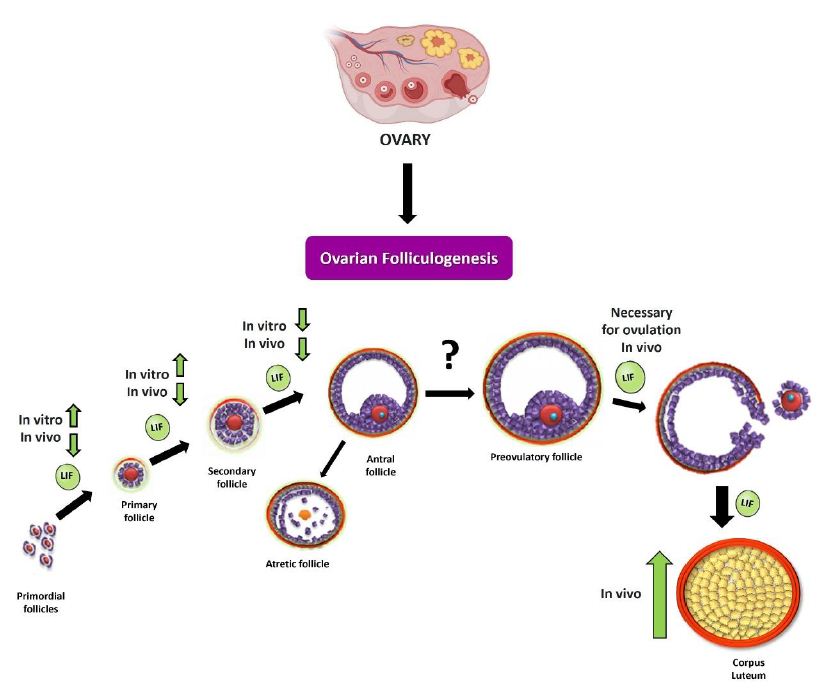
Figure 2: Summary scheme of the effects of LIF on ovarian folliculogenesis. As discussed in the review, LIF can modulate the different stages of ovarian folliculogenesis in vitro and in vivo. During initial recruitment (passage from primordial follicle to preantal follicle), LIF promotes recruitment by increasing the number of developing follicles (primary and secondary follicles) in an in vitro neonatal ovary model [43]. In the fertile stage, LIF produces a decrease in the number of preantral follicles, in an in vivo model [48]. In studies carried out in the prepubertal stage [27] and in the fertile stage, it is observed that LIF decreases the size and number of antral follicles. It has also been observed that LIF is necessary for ovulation to occur [41], which could be closely related to the increase in the number of large corpora lutea following chronic treatment with this cytokine.
References
- Abir R, Fisch B, Jin S, Barnnet M, Freimann S, van den Hurk R, Feldberg D, Nitke S, Krissi H, Ao A, et al. (2004) Immunocytochemical detection and RT-PCR expression of leukaemia inhibitory factor and its receptor in human fetal and adult ovaries. Molecular Human Reproduction 10: 313-319. [crossref]
- Acuña E, Fornes R, Fernandois D, Garrido MP, Greiner M, Lara HE, Paredes AH, et al. (2009) Increases in norepinephrine release and ovarian cyst formation during ageing in the rat. Reproductive Biology and Endocrinology 7. [crossref]
- Aikawa J, Sato E, Kyuwa S, Sato E, Sasai K, Shiota K, Ogawa T, et al. (1998) Asparagine-linked glycosylation of the rat leukemia inhibitory factor expressed by simian COS7 cells. Bioscience, Biotechnology, and Biochemistry 62: 1318-1325. [crossref]
- Akita S, Webster J, Ren SG, Takino H, Said J, Zand O, Melmed S (1995) Human and Murine Pituitary Expression of Leukemia Inhibitory Factor: Novel Intrapituitary Regulation of Adrenocorticotropin Hormone Synthesis and Secretion. Journal of Clinical Investigation 95: 1288-1298. [crossref]
- Arici A, Oral E, Bahtiyar O, Engin O, Seli E, Jones EE, et al. (1997) Leukaemia inhibitory factor expression in human follicular fluid and ovarian cells. Human Reproduction Vol 12: 1233-1239. [crossref]
- Fukada K, Korsching S, Towle MF (1997) Tissue-specific and ontogenetic regulation of LIF protein levels determined by quantitative enzyme immunoassay. Growth Factors 14: 279-295. [crossref]
- Auernhammer CJ, Melmed S (2000) Leukemia-Inhibitory Factor-Neuroimmune Modulator of Endocrine Function. Endocrine Reviews 21: 313-345. [crossref]
- Bamberger A, Schulte H, Wullbrand A, Jung R, Beil F, Bamberger C (2000) Expression of leukemia inhibitory factor (LIF) and LIF receptor (LIF-R) in the human adrenal cortex: implications for steroidogenesis. Molecular and Cellular Endocrinology 162: 145-149. [crossref]
- Bazan JF (1991) Neuropoietic Cytokines in the Hematopoietic Fold. Neuron 7: 197-208. [crossref]
- Bhatt H, Brunet LJ, Stewart CL (1991) Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Developmental Biology 88: 11408-11412. [crossref]
- Calder M, Chan YM, Raj R, Pampillo M, Elbert A, Noonan M, Gillio-Meina C, Caligioni C, Bérubé NG, Bhattacharya M, et al. (2014) Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology 155: 3065-3078. [crossref]
- Chun S-Y, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJW (1996) Hormonal Regulation of Apoptosis in Early Antral Follicles: Follicle-Stimulating Hormone as a Major Survival Factor*. Endocrinology 137: 1447-1456. [crossref]
- Coskun S, Uzumcu M, Jaroudi K, Hollanders JMG, Parhar RS, Al-Sedairy ST (1998) Presence of leukemia inhibitory factor and interleukin-12 in human follicular fluid during follicular growth. American Journal of Reproductive Immunology 40: 13-18. [crossref]
- Curley M, Milne L, Smith S, Atanassova N, Rebourcet D, Darbey A, Hadoke PWF, Wells S, Smith LB, et al. (2018) Leukemia Inhibitory Factor-Receptor is Dispensable for Prenatal Testis Development but is Required in Sertoli cells for Normal Spermatogenesis in Mice. Scientific Reports 8: 1-13. [crossref]
- Davis JS, Rueda BR (2002) The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Frontiers in Bioscience 7: 1949-1978. [crossref]
- Dong J, Guo C, Zhou S, Zhao A, Li J, Mi Y, Zhang C (2022) Leukemia inhibitory factor prevents chicken follicular atresia through PI3K/AKT and Stat3 signaling pathways: LIF prevents chicken follicular atresia. Molecular and Cellular Endocrinology 543: 5789-5796. [crossref]
- Dozio E, Ruscica M, Galliera E, Corsi MM, Magni P (2009) Leptin, Ciliary Neurotrophic Factor, Leukemia Inhibitory Factor and In-terleukin-6: Class-I Cytokines Involved in the Neuroendocrine Regulation of the Reproductive Function. Current Protein and Peptide Science 10: 577-584. [crossref]
- Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R (2013) P42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cellular Signalling 25 898-909. [crossref]
- Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, Nice EC, Kelso A, Metcalf D (1987) Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF) The EMBO Journal 6 3995-4002. [crossref]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA (2004) A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145 4073-4077. [crossref]
- Harter CJL, Kavanagh GS, Smith JT (2018) The role of kisspeptin neurons in reproduction and metabolism. Journal of Endocrinology 238 R173-R183. [crossref]
- Hilton DJ (1992) LIF: lots of interesting functions. Trends in Biochemical Sciences 17 72-76. [crossref]
- Hinds MG, Maurer T, Zhang JG, Nicola NA, Norton RS (1998) Solution structure of leukemia inhibitory factor. Journal of Biological Chemistry 273 13738-13745. [crossref]
- Ilha GF, Rovani MT, Gasperin BG, Antoniazzi AQ, Gonçalves PBD, Bordignon V, Duggavathi R (2015) Lack of FSH support enhances LIF-STAT3 signaling in granulosa cells of atretic follicles in cattle. Reproduction 150 395-403. [crossref]
- Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, et al. (2010) Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. Journal of Clinical Investigation 120 408-421. [crossref]
- Keltz MD, Attar E, Buradagunta S, Olive DL, Kliman H, Arici A (1996) Modulation of leukemia inhibitory factor gene expression protein biosynthesis in the human fallopian tube. American Journal of Obstetrics and Gynecology 175 1611-1619. [crossref]
- Komatsu K, Koya T, Wang J, Yamashita M, Kikkawa F, Iwase A (2015) Analysis of the effect of leukemia inhibitory factor on follicular growth in cultured murine ovarian tissue. Biology of Reproduction 93 1-18. [crossref]
- Lass A, Weiser W, Munafo A, Loumaye E (2001) Leukemia inhibitory factor in human reproduction. Fertility and Sterility 76 1091-1096.
- Lecomte MJ, de Gois S, Guerci A, Ravassard P, Biguet NF, Mallet J, Berrard S (2005) Differential expression and regulation of the high-affinity choline transporter CHT1 and choline acetyltransferase in neurons of superior cervical ganglia. Molecular and Cellular Neuroscience 28 303-313. [crossref]
- Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Taieb J, Moreau JF, Chaouat G (2001) Follicular fluid concentration of leukaemia inhibitory factor is decreased among women with polycystic ovarian syndrome during assisted reproduction cycles. Human Reproduction 16 2073-2078. [crossref]
- Li Z, Zhu Y, Li H, Jiang W, Liu H, Yan J, Chen ZJ, Li W (2018) Leukaemia inhibitory factor in serum and follicular fluid of women with polycystic ovary syndrome and its correlation with IVF outcome. Reproductive BioMedicine Online 36 483-489. [crossref]
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L (2007) Is human chorionic gonadotropin directly involved in the regulation of human implantation? Molecular and Cellular Endocrinology 269 85-92. [crossref]
- Lovejoy B, Cascio D, Eisenberg D (1993) Crystal structure of canine and bovine granulocyte-colony stimulating factor (G-CSF) Journal of Molecular Biology 234 640-653. [crossref]
- Shen MM, Leder P (1992) Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proceedings of the National Academy of Sciences 89 8240-8244. [crossref]
- Magni P, Dozio E, Ruscica M, Watanobe H, Cariboni A, Zaninetti R, Motta M, Maggi R (2007) Leukemia inhibitory factor induces the chemomigration of immortalized gonadotropin-releasing hormone neurons through the independent activation of the Janus kinase/signal transducer and activator of transcription 3, mitogen-activated protein kinase/extracellularly regulated kinase 1/2, and phosphatidylinositol 3-kinase/Akt signaling pathways. Molecular Endocrinology 21 1163-1174. [crossref]
- McDonald NQ, Panayotatos N, Hendrickson WA (1995) Crystal structure of dimeric human ciliary neurotrophic factor determined by MAD phasing. EMBO Journal 14 2689-2699. [crossref]
- Mcgee EA, Hsueh AJW (2000) Initial and Cyclic Recruitment of Ovarian Follicles. Endocrine Reviews 21 200-214. [crossref]
- Meng L, Jan SZ, Hamer G, van Pelt AM, van der Stelt I, Keijer J, Teerds KJ (2018) Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biology of Reproduction 99 853-863. [crossref]
- Mikhaylova IV, Jääskeläinen T, Jääskeläinen J, Palvimo JJ, Voutilainen R (2008) Leukemia inhibitory factor as a regulator of steroidogenesis in human NCI-H295R adrenocortical cells. Journal of Endocrinology 199 435-444. [crossref]
- Mo X, Wu G, Yuan D, Jia B, Liu C, Zhu S, Hou Y (2014) Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Molecular Reproduction and Development 81 608-618. [crossref]
- Murphy MJ, Halow NG, Royer PA, Hennebold JD (2016) Leukemia inhibitory factor is necessary for ovulation in female rhesus macaques. Endocrinology 157 4378-4387. [crossref]
- Nicola NA, Babon JJ (2015) Leukemia inhibitory factor (LIF) Cytokine and Growth Factor Reviews 26 533-544.
- Nilsson E, Kezele P, Skinner MK (2002) Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Molecular and Cellular Endocrinology 188 65-73. [crossref]
- da Nóbrega JE, Gonçalves PBD, Chaves RN, Magalhães DDM, Rossetto R, Lima-Verde IB, Pereira GR, Campello CC, Figueiredo JR, de Oliveira JFC (2012) Leukemia inhibitory factor stimulates the transition of primordial to primary follicle and supports the goat primordial follicle viability in vitro. Zygote 20 73-78. [crossref]
- Palmqvist P, Persson E, Conaway HH, Lerner UH (2002) IL-6, Leukemia Inhibitory Factor, and Oncostatin M Stimulate Bone Resorption and Regulate the Expression of Receptor Activator of NF-κB Ligand, Osteoprotegerin, and Receptor Activator of NF-κB in Mouse Calvariae. The Journal of Immunology 169 3353-3362. [crossref]
- Pastuschek J, Poetzsch J, Morales-Prieto DM, Schleußner E, Markert UR, Georgiev G (2015) Stimulation of the JAK/STAT pathway by LIF and OSM in the human granulosa cell line COV434. Journal of Reproductive Immunology 108 48-55. [crossref]
- Pawar S, Starosvetsky E, Orvis GD, Behringer RR, Bagchi IC, Bagchi MK (2013) STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Molecular Endocrinology 27 1996-2012. [crossref]
- Peña S, Rubio M, Vargas C, Alanis C, Paredes A (2023) Participation of leukaemia inhibitory factor in follicular development and steroidogenesis in rat ovaries. Journal of Endocrinology 258. [crossref]
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M (2012) Kisspeptins and Reproduction: Physiological Roles and Regulatory Mechanisms. Physiol Rev 92 1235-1316. [crossref]
- Reynaud K, Driancourt MA (2000) Oocyte attrition. Molecular and Cellular Endocrinology 163 101-108. [crossref]
- Robinson RC, Grey LM, Staunton D, Vankelecom H, Vernallis AB, Moreau J-F, Stuart DI, Heath JK, Jones EY (1994) The Crystal Structure and Biological Function of Leukemia Inhibitory Factor: Implications for Receptor Binding. Cell. [crossref]
- Sasai K, Aikawa J, Saburi S, Tojo H, Tanaka S, Ogawa T, Shiota K (1998) Functions of the N-Glycans of Rat Leukemia Inhibitory Factor Expressed in Chinese Hamster Ovary Cells. Biochem 124 999-1003. [crossref]
- Senturk LM, Arici A (1998) Leukemia inhibitory factor in human reproduction. American Journal of Reproductive Immunology 39 144-151. [crossref]
- Sims NA, Johnson RW (2012) Leukemia inhibitory factor: A paracrine mediator of bone metabolism. Growth Factors 30 76-87. [crossref]
- Somers W, Stahl M, Seehra JS (1997) 1.9 Å crystal structure of interleukin 6: Implications for a novel mode of receptor dimerization and signaling. EMBO Journal 16 989-997. [crossref]
- Stewart C (1994) Leukaemia inhibitory factor and the regulation of pre-implantation development of the mammalian embryo. Molecular Reproduction and Development 39 233-238. [crossref]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359 76-79. [crossref]
- Sun X, Bartos A, Whitsett JA, Dey SK (2013) Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Molecular Endocrinology 27 1492-1501. [crossref]
- Taupin J-L, Pitard V, Dechanet J, Miossec V, Gualde N, Moreau J-F (1998) Leukemia Inhibitory Factor: Part of a Large Ingathering Family. International Reviews of Immunology 16 397-426. [crossref]
- Ultsch MH, Somers W, Kossiakoff AA, de Vos A (1994) The crystal structure of affinity-matured human growth hormone at 2 Å resolution. Journal of Molecular Biology 236 286-299. [crossref]
- Viswanadhapalli S, Dileep KV, Zhang KYJ, Nair HB, Vadlamudi RK (2022) Targeting LIF/LIFR signaling in cancer. Genes and Diseases 9 973-980. [crossref]
- Wang Y, Yuan K, Li X, Su Z, Li X, Guan H, Su Y, Ge HS, Ge RS (2016) Leukemia inhibitory factor stimulates steroidogenesis of rat immature Leydig cells via increasing the expression of steroidogenic acute regulatory protein. Growth Factors 34 166-176. [crossref]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann M, Patterson PH (1989) The Cholinergic Neuronal Differentiation Factor from Heart Cells Is Identical to Leukemia Inhibitory Factor. Natl. Acad. Sci. U.S.A 86 1384. [crossref]
- Yu-Lan Chu, Ya-Ru Xu, Wan-Xi Yang, Yi Sun (2018) The role of FSH and TGF-β superfamily in follicle atresia. Aging (Albany NY) 10 305-321. [crossref]
- Zgheib C, Zouein FA, Kurdi M, Booz GW (2012) Chronic treatment of mice with leukemia inhibitory factor does not cause adverse cardiac remodeling but improves heart function. European Cytokine Network 23 197.
- Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, Dimarchi RD, Furman TC, Hale JE, Hsiung HM, et al. (1997) Crystal structure of the obese protein leptin-E100. Nature 287 206-209. [crossref]