Abstract
Desensitising products designed for use in the treatment of Dentine Hypersensitivity (DH) are available either through in-office procedures (professional products) or direct to the consumer (over the counter products [OTC]). This paper is an overview on selected OTC products available to the consumer and compares the reported effectiveness of the different active ingredients present in these products. Information was collected from several sources including direct observation of the toothpastes available in a UK supermarket and from online retailers (such as Amazon etc.,) as well as reviewing the published evidence from randomised control trials, systematic reviews, and meta-analyses as well as clinical studies. A comparison of the claims of effectiveness in reducing Dentine Hypersensitivity by toothpaste manufacturers on the toothpaste cartons (e.g., packaging and labelling) was compared with the results from these peer reviewed publications. Evidence from these publications would suggest that products containing potassium, stannous fluoride, calcium sodium phosphor-silicate, arginine, nano-hydroxyapatite, and fluoro-calcium-phospho-silicate ingredients have sufficient evidence to support their effectiveness in managing DH. There is, however, contradicting information on the effectiveness of potassium containing products in the published literature.
Introduction
Dentine Hypersensitivity (DH) is a somewhat puzzling clinical condition which may impact on the Quality of Life (QoL) of those who suffer from the problem [1,2]. The pain associated with DH has been described as ‘rapid in onset, sharp in character and transient in nature’, and will resolve once the offending stimulus has been removed [23]. The prevalence of DH varies depending on how data is collected, for example, questionnaire values range from 4% to 74% whereas clinical studies would suggest lower values in the region of 11.5% [3]. These higher values may suggest that self-reporting of DH may be exaggerated compared to clinical results as well as variations in the different populations that were assessed. From an epidemiological perspective, there was a slightly higher prevalence in females than in males which was not statistically significant. Evidence from clinical evaluation would suggest, in the main, a lower prevalence value compared to the self- reported values by participants which may be due in part to the participants being unable to distinguish the various conditions associated with dental pain (e.g., toothache etc.).The underlying mechanism of DH is hydrodynamic in nature and based on the Hydrodynamic Theory where minute fluid shifts in the dentinal tubules initiates a pain response [4]. Currently most treatment approaches are based on this theory and as such most desensitising products (In-office professionally applied and/or over the counter (OTC)) are based on their tubular occluding properties [5]. The choice of recommending a product will depend on a clinician’s clinical judgement on the extent and severity of the clinical problem. The treatment of DH, however, is based on a correct diagnosis of the problem and by excluding all other possible causes of the individual’s discomfort (essentially DH is a diagnosis of exclusion), the choice of product or technique based on the extent and severity of the condition, patient compliance, and successful monitoring/ management of the problem over time. The aim of this short overview is therefore to evaluate the claims of effectiveness of selected over the counter desensitising products (packaging claims) and compare these claims with evidence from the available published literature.
Methodology
A study was conducted by one of the authors (HS) to identify a range of home or consumer (over the counter) desensitising toothpaste products for the treatment of DH in a local supermarket store in the UKas well as online retail websites (e.g., Amazon). Information relating to the ingredients of the various selected toothpastes together with the claims made on the cartons (packaging/labelling) which subsequently included data from the internet (manufacturers’ websites). A comparison was made on the various claims made by the manufacturers on their products with the available evidence from peer reviewed journals. This information was subsequently collated into tables as shown below (Tables 1 and 2). From the various desensitising products identified in the initial survey it was decided to concentrate on four selected products based a specific active ingredient namely: 1) Fluoro-Calcium-Phospho-Silicate (FCPS)(BioMin-F), 2) Stannous fluoride (Sensodyne Rapid Relief), 3) Arginine (Colgate Sensitive Instant Relief) and 4) Nano-hydroxyapatite (Curaprox Be you). A Potassium containing product with without other active ingredients (e.g., stannous fluoride or hydroxyapatite) together with a Calcium Sodium Phospho-silicate (CSP) product (e.g. Novamin based) was included when. discussing the results (Table 2).
Table 1: Selected over the counter desensitising toothpaste products available in the UK consumer market with their listed ingredients and their packaging claims.
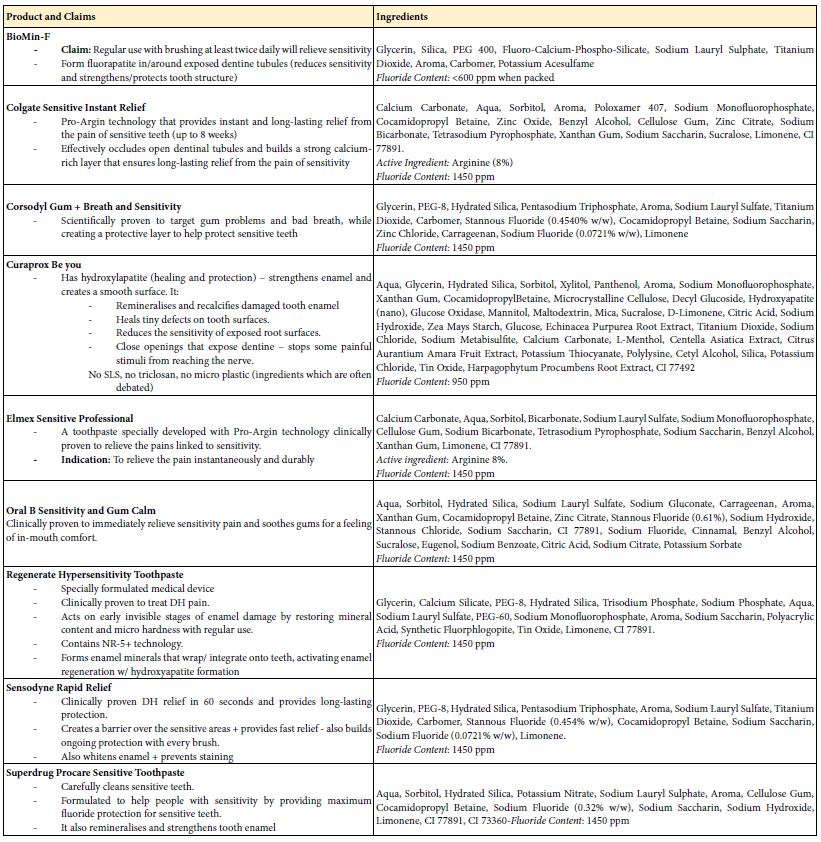
Products on the Market
The following selected products were identified in an initial survey (Table 1):
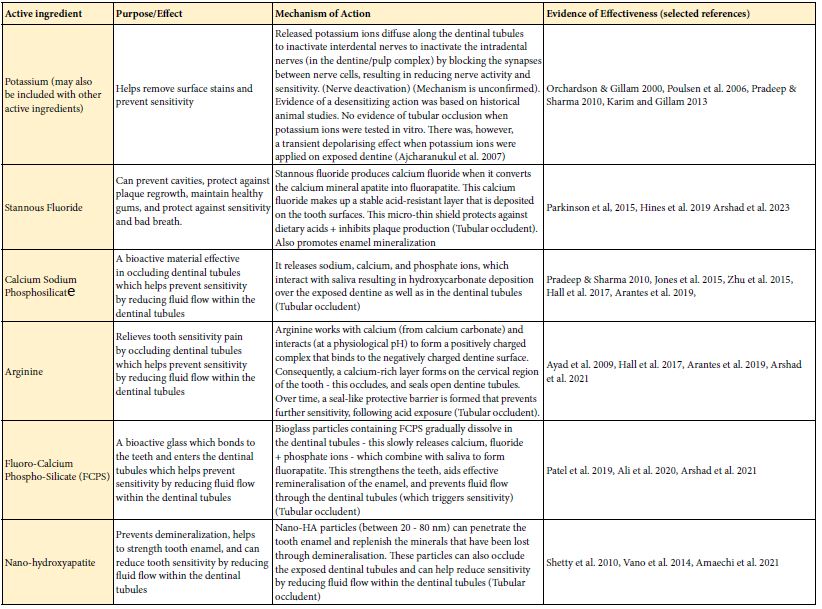
The following table (Table 2) highlights the purpose/aim and mechanism of action for each toothpaste and their main ingredient(s) together with the supporting evidence from the published literature.
Table 2: The purpose/aim and mechanism of action for each toothpaste and their main ingredient(s)
Discussion
According to Vranić et al. [6] the main components of a toothpaste are abrasives, humectants, surfactants, binders, and flavouring agent together with any active ingredients. The formulation of toothpaste products is a complex procedure, and it is essential to ensure that any of the other ingredients within the formulation do not impact with the delivery of the active ingredient. According to Rathore and Gillam [7] most manufacturers, make claims under the Cosmetic regulations rather than making a direct clinical claim such as ‘prevents gingivitis’ etc., which would require clinical evidence from well-conducted randomised clinical trials (RCT) to claim clinical efficacy [8]. For this selected overview on over the counter (OTC) desensitising products, examples of the various active ingredients (initially identified from the consumer brands in a UK supermarket and online retail websites), together with their respective claims of effectiveness were assessed from the available published literature (which included evidence from systematic, reviews, meta-analysis, reviews [including Cochrane Reviews] and clinical studies).
One of the problems in evaluating the evidence for these studies was 1) the lack of homogeneity between the studies particularly with view to the length of duration and assessment methodology of the selected studies for example reviews based a Cochrane type of review would only include studies of a minimum of six weeks duration as well as including similar ingredients in both control and test groups [9-11], 2) the likelihood that some of the active ingredients within the formulation may have changed over the decades and 3) the problems of the highly subjective nature when reporting Dentine Hypersensitivity.
Based on the evidence from the published literature it can be concluded that the active ingredients outlined in Tables 1 and 2 have been shown to be effective in reducing DH [12-16]. However, there is clearly a need to have well controlled clinical studies on a duration that is relevant to the claims being made. For example, if a short acting or immediate (rapid) effect is claimed than the time intervals in the study should reflect this (e.g., retesting within 5-10 minutes following application). Alternatively, if a long-lasting effect is claimed than the time intervals should reflect this (e.g., 3-6 months), furthermore if claims of protection against ‘acid erosion’ are made than evidence from in vitro or in situ studies should support this. It should be acknowledged, however that some toothpaste formulations may take longer to be effective in reducing DH. From a clinical perspective it may be reasonable for the clinician and patient to accept that the discomfort from DH may not be eliminated but there is some relief that enables them to enjoy a better Quality of Life (QoL). According to Rathore & Gillam [7] one of the advantages of publishing these claims on the packaging is that this may enable the consumer to identify an OTC product that is relevant to their specific needs such as a recommended toothpaste for ‘sensitivity’ with a degree of confidence that the product may actually help them in resolving their problem [17-32].
Conclusion
There appears that the conclusions from the published literature acknowledge the effectiveness of selected OTC desensitising toothpaste products e.g., potassium, stannous fluoride, calcium sodium phosphosilicate (CSP) and arginine have sufficient supporting evidence to justify their claims. Evidence to support the use of nano- hydroxyapatite (nano-HA) and Fluoro-Calcium-Phospho-Silicate (FCPS) is growing and some studies have shown that they have a similar or improved effect on reducing DH than the other ingredients. There is, however, contradictory evidence regarding the effectiveness of the potassium ion in potassium-based toothpastes.
Disclaimer
One of the Authors (DG) has several patents on oral care products and currently is a Director with Biomin Technology Limited, UK. There was, however, no commercial involvement in preparing and writing up the research undertaken in this study.
References
- Gillam DG (2013) Current diagnosis of dentin hypersensitivity in the dental office: an Clin Oral Investig. 17 Suppl 1(Suppl 1): S21-S29. [crossref]
- Cunha-Cruz J, Wataha JC (2014) The burden of dentine In Dentine Hypersensitivity: Developing a Person-centred Approach to Oral Health Robinson PG (ed), pp 34-44.
- Favaro ZL, Soares PV, Cunha-Cruz (2019) Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J Dent. 81: 1-6. [crossref]
- Brännström M, Åström A (1972) The hydrodynamics of the dentin; its possible relationship to dentinal pain. International Dental Journal 22: 219-227. [crossref]
- Gillam DG (2017) A New Perspective on Dentine Hypersensitivity – Guidelines for General Dental Practice. Dent Update. 44: 33-6, 39-42. [crossref]
- Vranić E, Lacević A, Mehmedagić A, Uzunović A (2004) Formulation ingredients for toothpastes and Bosn J Basic Med Sci. 4: 51-8. [crossref]
- Rathore M,Gillam DG (2024) The Effectiveness of Selective Toothpaste Ingredients and Formulations in The Treatment of Gum Health- A Review. Clin Oral Sci Dent (2024), 7:1.
- CTPA Guide on Classification of Toothpaste Claims Borderline issues between Cosmetics and Medicinal Products or medical devices Common Understanding (2023) NEW CTPA Guide on Common Understanding of Borderline Toothpaste Claims.
- Orchardson R, Gilam DG. (2000) ‘The efficacy of potassium salts as agents for treating dentin hypersensitivity’. Journal of Orofacial Pain. 14: 9-19. [crossref]
- Poulsen S, Errboe M, Lescay MY, Glenny AM (2006) Potassium containing toothpastes for dentine Cochrane Database Syst Rev. 19: CD001476. [crossref]
- Karim BFA,Gillam DG (2013) The Efficacy of Strontium and Potassium Toothpastes in Treating Dentine Hypersensitivity: A Systematic Review International Journal of Dentistry. [crossref]
- Bae HJ. Kim KY,Myung KS. (2015) ‘Desensitizing toothpaste versus placebo for dentin hypersensitivity: a systematic review and meta-analysis’, Journal of Clinical Periodontology 42: 131-41. [crossref]
- Jones BS, Parkinson RC, Jeffery P, Davies M, Macdonald L E., et al. (2015) A randomised clinical trial investigating calcium sodium phosphosilicate as a dentine mineralising agent in the oral environment, Journal of Dentistry 43: 757-64. [crossref]
- Marto CM, Baptista PA, Nunes T, Pimenta M, Abrantes AM, et (2019) Evaluation of the efficacy of dentin hypersensitivity treatments-A systematic review and follow- up analysis. J Oral Rehabil. 46: 952-990. [crossref]
- Martins CC, Firmino RT, Riva JJ, Ge L, Carrasco-Labra , et al (2020) Desensitizing Toothpastes for Dentin Hypersensitivity: A Network Meta-analysis. Journal of Dental Research 99: 514-22. [crossref]
- Martins CC, Riva JJ, Firmino RT, Schünemann HJ (2022) Formulations of desensitizing toothpastes for dentin hypersensitivity: a scoping review. J Appl Oral 7: 30: e20210410. [crossref]
- Ali S, Farooq I, Al-Thobity AM, Al-Khalifa KS, Alhooshani K, et (2020) An in- vitro evaluation of fluoride content and enamel remineralization potential of two toothpastes containing different bioactive glasses. Biomed Mater Eng. 30: 487-496. [crossref]
- Ajcharanukul O, Kraivaphan P, Wanachantararak S, Vongsavan N, Matthews B (2007) Effects of potassium ions on dentine sensitivity in man. Arch Oral Biol. 52: 632-9. [crossref]
- Amaechi BT, Lemke KC, Saha et al. (2021) Clinical efficacy of nanohydroxyapatite- containing toothpaste at relieving dentin hypersensitivity: an 8 weeks randomized control trial. BDJ Open 7: 23 (2021) [crossref]
- Arantes DC, Limeira FIR, Yamauti M, Moreira AN, Abreu LG, et al. (2019) Comparison of Clinical Efficacy of Pro-Argin and NovaMin Toothpastes in Relieving Dentin Hypersensitivity: A Systematic Review and Meta-analysis. Oral Health Prev Dent. 17: 403-412. [crossref]
- Arshad S, Zaidi AJS, Farooqui AW. (2021) Comparative efficacy of BioMin-F, Colgate Sensitive Pro-relief and Sensodyne Rapid Action in relieving dentin hypersensitivity: a randomised controlled trial, BioMedical Centre 21: 498. [crossref]
- Ayad F, Ayad N, Delgado E, Zhang YP, DeVizio W, et al. (2009) Comparing the efficacy in providing instant relief of dentin hypersensitivity of a new toothpaste containing 0% arginine, calcium carbonate, and 1450 ppm fluoride to a benchmark desensitizing toothpaste containing 2% potassium ion and 1450 ppm fluoride, and to a control toothpaste with 1450 ppm fluoride: a three-day clinical study in Mississauga, Canada. J Clin Dent. 20: 115-22. [crossref]
- Canadian Advisory Board on Dentin Hypersensitivity Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity (2003) J Can Dent Assoc. 69: 221–226.
- Hall C, Mason S, Cooke (2017) Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes – a 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste- for the longer-term relief of dentine hypersensitivity. J Dent. 60: 36-43. [crossref]
- Hines D, Xu S, Stranick M, Lavender S, Pilch S, et al. (2019) Effect of a stannous fluoride toothpaste on dentinal hypersensitivity: In vitro and clinical J Am Dent Assoc. 150: S47-S59. [crossref]
- Parkinson CR, Jeffery P, Milleman JL, Milleman KR, Mason S (2015) Confirmation of efficacy in providing relief from the pain of dentin hypersensitivity of an anhydrous dentifrice containing 0.454% with or without stannous fluoride in an 8-week randomized clinical trial. Am J Dent. 28: 190-6. [crossref]
- Patel VR, Shettar L, Thakur S, Gillam D, Kamala DN (2019) A randomised clinical trial on the efficacy of 5% fluorocalcium phosphosilicate-containing novel bioactive glass J Oral Rehabil. 46: 1121-1126. [crossref]
- Pradeep AR,Sharma A (2010) Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: a randomized clinical trial. Periodontol. 81: 1167-1173. [crossref]
- Shetty S, Kohad R, Yeltiwar R (2010) Hydroxyapatite as an in-office agent for tooth hypersensitivity a clinical and scanning electron microscopic J. Periodontol. 81: 1781–1789. [crossrefhttps: //pubmed.ncbi.nlm.nih.gov/20681811/]
- Vano M, Derchi G, Barone A, Covani U (2014) Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: a double-blind randomized controlled Quintessence Int. 45: 703-11. [crossref]
- West XN, Seong J,Davies (2015) Management of dentine hypersensitivity: efficacy of professionally and self-administered agents, Journal of Clinical Periodontology, 256-302. [crossref]
- Zhu M, Li J, Chen B, Mei L, Yao L, et al. (2015) The Effect of Calcium Sodium Phosphosilicate on Dentin Hypersensitivity: A Systematic Review and Meta- PLoS One. 10: e0140176.