DOI: 10.31038/CST.2018312
Abstract
An overlap clinical and biochemical can occur between dogs with sudden acquired retinal degeneration syndrome (SARDS) and those with blindness by hyperadrenocorticism (HAC). A 13-year-old, cross-breed female dog showing recent signs of polyphagia, swollen abdomen and sudden blindness was examined. The flat electroretinography was consistent with bilateral retinal damage. The biochemical tests for the diagnosis of HAC were inconclusive when the blindness was established. However, HAC was confirmed two months after through the urine cortisol: creatinine ratio (UCCR: 69.4 × 10-6, RV: <10), plasmatic cortisol 1-hour post-ACTH (21.7 µg/dl, RV: <17) and 8-hour post-low dose dexamethasone suppression test (LDDST: 2.9 µg/dL, RV: <1.5). A basophilic microadenoma of the adenohypophysis (2.3 x 1.3 mm) with immunopositivity for ACTH was identified by histopathology. The final diagnosis was pituitary-dependent hyperadrenocorticism (PDH). This report, demonstrates through histopathology the association between a corticotropinoma and the sudden blindness by retinal damage in a dog with Cushing’s syndrome. In addition, it highlights the importance of timely use of the various hormonal tests for the correct diagnosis of HAC.
Keywords
ACTH, electroretinography, hyperadrenocorticism, pituitary, SARDS
Introduction
Sudden blindness with normal ophthalmoscopy and absence of electroretinography (ERG) response may be seen in dogs with PDH, but also with SARDS [1-4]. Likewise, dogs with SARDS may display systemic signs of HAC such as polyphagia (PF), polyuria (PU), polydipsia (PD), weight gain and biochemical changes (e.g., increase in serum alkaline phosphatase [SAP], total cholesterol [TChol]) in more than 80% of cases [3,4]. In the light of these arguments, there is a clear clinical and biochemical overlap between these two entities where blindness is irreversible [4]. In PDH cases, non-treatment may lead to the onset of numerous comorbidities (e.g., diabetes mellitus, hypertension, dyslipidemia) that can dramatically decrease survival [3, 5]. The confirmation or exclusion of its diagnosis is therefore essential.
This case offers the first histopathological evidence of the connection between a functional corticotropinoma (micro-adenoma) and sudden blindness caused by retinopathy.
Case report
A 13-year-old, cross-breed, neutered female dog weighing 12.5 Kg was referred for evaluation for sudden blindness. Additionally, it suffered from PF (two weeks before blindness), muscle atrophy in limbs, swollen abdomen and excessive panting (Figure 1). Negative response to threat test, slightly mydriatic pupils, photomotor reflex negative response, normal fundus with intraocular normotension and a flat ERG were consistent with retinal injury (Figure 2). Increased SAP (450 UI/ml, RV: < 250), TChol (250 mg/dl, RV: < 220) and decreased urine density (1022, RV: > 1030) were the only changes in the biochemistry and the hemogram (Table 1). Slightly increased UCCR with plasmatic cortisol suppression 8 hours post LDDST and normal adrenal glands in the ultrasonography ruled out HAC on the first examination (Table 1).
Table 1. Biochemical diagnosis and follow-up of Cushing disease
|
Parameter |
Day |
Reference |
||
|
1 |
60 |
120* |
Values |
|
| Adrenal axis evaluation | ||||
|
UCCRa/b |
13.7 |
69.4 |
42.3 |
<10 |
|
Coa |
5.2 |
– |
– |
1.0-4.5 |
|
Co4h-LDDSTa |
<1.0 |
– |
– |
<1.5 |
|
Co8h-LDDSTa |
1.4 |
2.9 |
– |
< 1.5 |
|
Co1h-ACTHa |
– |
21.7 |
– |
<17 |
|
pACTHa |
– |
48 |
– |
5-60 |
|
SAPb |
450 |
498 |
309 |
<250 |
|
BAW |
6.8 |
7.7 |
9.1 |
< 7.4 |
|
Metabolic profile |
|
|
|
|
|
TCholb |
250 |
322 |
221 |
< 220 |
|
Tgb |
77 |
173 |
102 |
< 120 |
|
Gb |
73 |
96 |
89 |
< 125 |
|
SP/DP |
145/90 |
160/95 |
150/80 |
<150/<90 |
|
W |
12.4 |
13.6 |
13.0 |
|
|
UPC |
0.2 |
0.7 |
0.3 |
< 0.3 |
UCCR: urine cortisol/creatinine ratio (x10-6); Co: plasma cortisol (µg/dl); Co4h-LDDST: Co at 4 hours post LDDST; Co8h-LDDST: Co at 8 hours post LDDST; Co1h-ACTH: Co at 1 hours post ACTH; pACTH: plasma adrenocorticotropic hormone (pg/ml); SAP: seric alkaline phosphatase (UI/l); BAW: bilateral adrenal width (mm); TChol: total colesterol (mg/dl); Tg: triglycerides (mg/dl); G: glucose (mg/dl); SP/DP: systolic and diastolic pressure (mmHg); W: weight (Kg); UPC: urinary protein creatinine; a: chemiluminescence; b: spectrophotometry; *: under treatment with Cabergoline and ketoconazole (0.07 mg/Kg PO q72h and 10 mg/kg PO q24h, respectively).
Two months later, in view of the development of PU, PD, compounded with weight gain, a second examination on the adrenal axis was performed, and the plasmatic cortisol 1-hour post-ACTH and 8-hour post-LDDST was found to be consistent with HAC (Table 1). Plasmatic ACTH (pACTH: 48 pg/ml, RV: 5-60) was found to be improperly high vis-a-vis the UCCR (69.4×10-6, RV: <10). Unlike the findings of the first examination, an increase in triglycerides (Tg), systolic pressure, diastolic pressure and the urine protein: creatinine ratio became evident (Table 1).
Based on the previous findings, PDH was deduced and cabergoline was then administered as treatment (0.07mg/Kg PO q72h) [6] and Ketoconazol (10 mg/kg PO q12h, tolerated dose) [3]. The biochemical and clinical follow-up performed two months later revealed a reduction of clinical signs (PU, PD and PF), the normalization of TChol, Tg, blood pressure, and a reduction of SAP and UCCR (Table 1). Likewise, hyperplasia of both adrenal glands was observed in the ultrasonography. Shortly afterwards, an accidental fall, favored by the blindness, resulted in a hip fractured that precipitated the humanitarian euthanasia of the animal. The owners authorized a transsphenoidal extraction of the pituitary and both adrenal glands by celiotomy. The extracted pituitary showed normal appearance and a whitish nodule on its outer edge (Figure 3), and both adrenal glands evidenced a size increase.
Normal adenohypophysis, in histological sections stained with hematoxylin-eosin (HE), consisted of a chain of globose-polyhedric cells arranged on a delicate fibrovascular stroma (Figure 4). In the periphery of the adenohypophysis, a proliferation of polyhedral cells organized in three to four rows of dense trabeculae of delicate fibrovascular stroma was found, which presented low pleomorphism, broad basophilic cytoplasm and a nucleus rounded with an evident nucleolus (Figure 5). These cells were organized in a larger, well-circumscribed, unencapsulated lobe, corresponding to an adenoma (2.34 x 1.3 mm) (Figure 6). The cells that make up the adenoma demonstrated intense positive cytoplasmic staining with the anti-ACTH antibody (mouse monoclonal, sc-52.980, Santa Cruz Biotechnology, Santa Cruz, CA, USA [1: 400 in PBS]) (Figure 7). Hiperplasia of the fascicular and reticular zones was found in both adrenal glands (Figure 8).
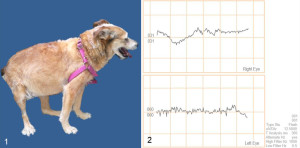
Figure 1. External aspect, bitch with pituitary-dependent hyperadrenocorticism and sudden blindness.
Figure 2. Electroretinography record showing flat waves in both eyes.
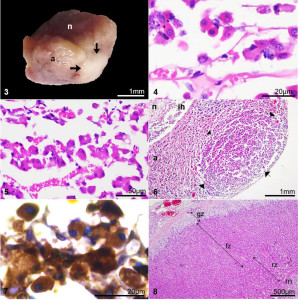
Figures 3-7. Pituitary, dog.
Figure 3. Macroscopic aspect of the pituitary and micro adenoma (arrows) after being fixed in 10% formaldehyde. n: neurohypophysis. a: adenohypophysis.
Figure 4. Microphotography normal adenohypophysis. A chain of globose-polyhedral cells, arranged in a delicate fibrovascular stroma. Hematoxylin-Eosin (H-E).
Figure 5. Microphotography basophilic adenoma composed of polyhedric cells with ample basophilic cytoplasm, arranged in thick trabeculae. H-E.
Figure 6. Microphotography pituitary with a basophilic adenoma of 2.3 x 1.3-mm (arrows) in adenohypophysis (a). ih: intermediate hypophysis. n: neurohypophysis. H-E.
Figure 7. Microphotography corticotropinoma cells with intense positive cytoplasmic staining with anti-ACTH antibody. Immunohistochemistry, Avidin-Biotin-peroxidase complex.
Figure 8. Microphotography adrenal cortex, diffuse hyperplasia fascicular zone (fz) and reticular zone (rz) with normal glomerular zone (gz). m: adrenal medulla (H-E).
Discussion
In this case, the sudden blindness, the absence of structural damage in both eyes, the integrity of optic chiasma (necropsy), the abolished ERG, the presence of systemic clinical signs (PF, PU, PD, weight gain) and the changes in biochemistry (SAP and TChol) were findings consistent with PDH, but also with SARDS [1,7]. The confirmation of PDH two months later the first evaluation using the same endocrine tests (LDDST and UCCR) was a peculiar finding. This fact highlights the advisability of performing a second endocrine evaluation in dogs with HAC signs and sudden blindness with inconcluse tests diagnostic, where a stimulation test should also be performed with ACTH [7], prior to reaching to any incorrect diagnosis of SARDS.
Images of the pitutary gland through computed tomography or magnetic resonance were no realizated; but the pituitary origin was deduced in view of plasma ACTH levels in connection with the increased UCCR and the absence of neoplastic injury to the adrenal glands. Additionally, it must be noted that even when using the CT and the MRI, pituitary tumors may not be detected in 28% to 37% of dogs with PDH [8,9]. Subsequently in the histopathology was confirmed the corticotropinoma. The basophilic staining characteristics obtained with the hematoxylin-eosin in the adenoma were compatible with the thyrotropic, gonadotropic or corticotropic cells, but the only cells ones of these that are immunopositive to anti-ACTH antibody are the corticotropic [10,11].
Like the blind dogs with PDH reported by Blatter [1], hypertriglyceridemia and hypercholesterolemia were found in this bitch in the second biochemical evaluation, but not at time of the onset of blindness. Other metabolic alterations mentioned in the retinopathy of dogs with Cushing’s disease are the increase in concentration of insulin, interleukin 6, and decrease in nitric oxide and adiponectin [2]; all of these except for insulinemia are not routinely used in the daily clinic, so their contribution to the development of blindness in this case could not be proven.
A limitation of this report is the retinal histopathological examination that could not be done due to conditions established by the owners in light of the need to extract both eyes.
Although the mechanism by which HAC induces sudden blindness in the dog is not well clarified, it is important to emphasize that its correct diagnosis and management of various comorbidities is fundamental to improve the survival of those dogs.
Finally, in dogs with sudden blindness, hypertension and dyslipidemia but with inconclusive diagnostic tests for HAC, it is recommended to carry out a timely medical and pharmacological management of each of the comorbidities, as well as a subsequent reevaluation of the adrenal axis.
References
- Cabrera Blatter MF, Del Prado B, Gallelli MF, D´Anna E, et al. (2012) Blindness in dogs with pituitary dependent hyperadrenocorticism: relationship with glucose, cortisol and triglyceride concentration and with ophthalmic blood flow. Res Vet Sci 387–392.
- Cabrera Blatter MF, Del Prado B, Miceli DD, Gomez N, et al. (2012) Interleukin-6 and insulin incrase and nitric oxide and adiponectin decrease in blind dogs with pituitary-dependent hyperadrenocorticism. Res Vet Sci 1195–1202.
- Feldman EC. Canine Hyperadrenocorticism (2015) In: Feldman EC, Nelson RW, Reusch CE, Scott-Moncrieff JCR, Behrend E. Canine & Feline Endocrinology, 4th ed. St. Louise, Missouri, USA: Elsevier, 377–451.
- Komáromy AM, Abrams KL, Heckenlively JR, Lundy SK, et al. (2016) Sudden acquired retinal degeneration syndrome (SARDS)–a review and proposed strategies toward a better understanding of pathogenesis, early diagnosis, and therapy. Vet Ophthalmol 19 : 319–31.
- Miceli DD, Pignataro OP, Castillo VA (2017) Concurrent hyperadrenocorticism and diabetes mellitus in dogs. Res Vet Sci 115: 425–431. [crossref]
- Castillo VA, Gómez NV, Lalia JC, Cabrera Blatter MF, García JD (2008) Cushing’s disease in dogs: cabergoline treatment. Res Vet Sci 85: 26–34. [crossref]
- Carter RT, Oliver JW, Stepien RL, Bentley E (2009) Elevations in sex hormones in dogs with sudden acquired retinal degeneration syndrome (SARDS). J Am Anim Hosp Assoc 45 : 207–14.
- Wood FD, Pollard RE, Uerling, Feldman EC (2007) Diagnostic imaging findings and endocrine test results in dogs with pituitary- dependent hyperadrenocorticism that did or did not have neurologic abnormalities: 157 cases (1989–2005). J Am Vet Med Assoc 231: 1081–1085.
- Auriemma E, Barthez PY, Van der Vlugt-Meijer RH, Voorhout G (2006) Computed tomography and low-field magnetic resonance imaging of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism: 11 cases (2001–2003). J Am Vet Med Assoc 235: 409–414.
- Jaime MN, López FIA, Cabrera GP (2003) Patología de los adenomas hipofisarios. Rev Esp Patol 36: 357–372.
- Thomas JR, Gröne A (2016) Pituitary glands. In: Jubb KBF, Kennedy PC, Palmer NC. Pathology of domestic animals, 6th ed. St. Louise, Missouri, USA: Elsevier 276–290.