Abstract
We revisit the formation of MC carbides in a model Ni-Co-Cr alloy containing carbon and the transition metal elements Hf, W, Ta, or Ti. We aim at illustrating that the thermodynamic stability of the MC carbides sensitively depends on the transition metal at case. All investigated alloys contain 1.15 at.% C and 1.20 at.% M (Hf, W, Ta, or Ti) being added to the baseline alloy Ni-31.3Co-31Cr (at.%). The selected carbon content is rather high and significantly higher compared to common superalloys, featuring carbide-reinforced alloys as potential candidates for laser based manufacturing. Thermodynamic computations using the Thermo-Calc software and the TCNi8 database show that only HfC is stable over the entire temperature range from room temperature up to the eutectic temperature of HfC formation, which is just above the solidus temperature of the Hf and C containing alloy.
Keywords
MC mono-carbides, Transition metal carbides, Thermodynamic computations, Carbide-reinforced alloys
Introduction
In most nickel-base alloys, transition metal mono-carbides (MC) with the crystal structure of NaCl [1] form in-situ during the late stages of solidification following a terminal eutectic reaction. In casting conditions, the MC carbides are therefore found in interdendritic regions only, quite often displaying the so-called Chinese script morphology [2-4]. These carbides are efficient to reduce grain boundary sliding during creep, but may deteriorate the tensile ductility and fatigue resistance by offering crack initiation sites and preferential crack propagation paths. Laser based processing, e.g. laser powder bed fusion, opens up new pathways for controlling the size and distribution of in-situ formed carbides. Recent articles have indeed reported that nano-sized and well-dispersed carbides are obtained by additive manufacturing in a number of Ni-based alloys [5-8], however the nature of the carbides is largely dependent on the alloy composition. This motivated us to revisit the thermodynamic stability of carbides in a model Ni-Co-Cr alloy containing carbon and the transition metal elements Hf, W, Ta, or Ti using thermodynamic computations with the software Thermo-Calc and the TCNi8 database [9]. In this short article, we will report on computed phase equilibria as function of temperature. In addition, we display the evolution of the solute content of Hf, W, Ta, or Ti in the solid solution matrix phase with the FCC face centered cubic crystal structure. We recall that phase equilibria are relevant for devising heat treatments and certainly for considerations of the phases in equilibrium with one another at the envisaged operation temperature. They are not sufficient for understanding phase evolution under the fast and directional solidification conditions inherent to laser processing.
Phase Equilibria and Thermodynamic Stability of MC Carbides
To address the question regarding the formation of carbides and the stability range of MC carbides we devised a generic alloy composition with carbon C and the transition metal M in nearly equimolar quantity (Table 1). Four alloys were defined accordingly; their composition in being listed in Table 1. For convenience we use wt.% instead of at.%.
Table 1: Overview of alloy compositions
|
Alloy composition |
Ni |
Co |
Cr |
C |
M = (Hf, Ta, W, Ti) |
| Generic (M), at.% |
35.35 |
31.3 | 31.0 | 1.15 |
1.20 M |
| Alloy 1 (Hf), wt.% |
36.0 |
32.0 |
28.0 |
0.24 |
3.72 Hf |
| Alloy 2 (W), wt.% |
36.0 |
32.0 |
28.0 |
0.24 |
3.83 W |
| Alloy 3 (Ta), wt.% |
36.0 |
32.0 |
28.0 |
0.24 |
3.77 Ta |
| Alloy 4 (Ti), wt.% |
37.0 |
32.9 |
28.8 |
0.25 |
1.03 Ti |
The computed phase equilibria are shown in the left-hand diagrams (a) of Figures 1-4, displaying the volume fraction of equilibrium phases as function of temperature. In all cases the low fraction range was chosen spanning from 0.0 to 0.2, i.e. from 0 to 20 vol.% The right-hand diagrams, labelled (b), display the corresponding evolution of the solute content inside the solid solution FCC phase, focusing on the element M, either Hf, W, Ta or Ti, by case.
Alloy 1 containing M=Hf in an amount of 1.2 at.% (3.72 wt.%) forms HfC from the last solidifying liquid (Figure 1a). The eutectic reaction LiquidFCC+HfC+Liquid’ extends over a rather wide temperature interval, which calls for special attention when applying laser-based additive manufacturing. The HfC is outstandingly stable over the widest temperature range down to room temperature. Below 700°C a trace amount of Cr23C6 forms in addition, but not at the expense of HfC. The Hf content in the FCC solid solution (Figure 1b) is very limited with a maximum value at T@1200°C as low as 0.2 at.% and gradually decreasing with decreasing temperature.
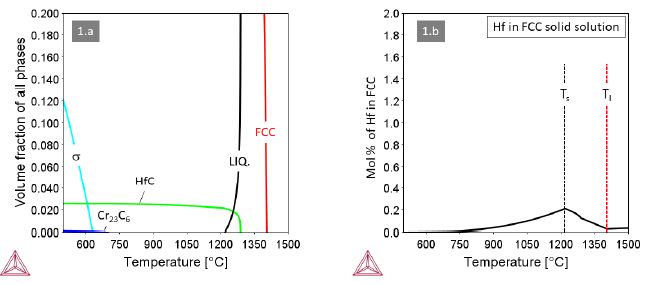
Figure 1: Thermodynamic equilibria in Alloy 1 with M=Hf
Alloy 2 containing M=W in an amount of 1.2 at.% (3.83 wt.%) forms Cr7C3 carbides from the very last solidifying liquid (Figure2a). All tungsten remains dissolved in the FCC solid solution. Around T@900°C the Cr7C3 carbide transforms to (Cr,W)23C6 along with a modest decrease of the W content in the solid solution. The R-phase Co27Cr18W8 stable below ~600°C finally consumes significant amounts of the dissolved W.
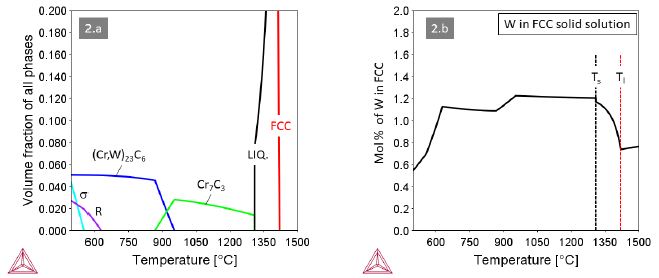
Figure 2: Thermodynamic equilibria in Alloy 1 with M=W
Alloys 3 and 4 with M=Ta and M=Ti, respectively, behave quite similar with MC and Cr7C3 carbides co-existing at high temperatures (Figures 3 and 4). A small difference relates to the carbide in the eutectic, being Cr7C3 in alloy 3 and TiC in alloy 4. Accordingly, the maximum solute content of Ta in the FCC solid solution is higher than that of Ti. In both alloys the Cr7C3 carbide transforms to (M,Cr)23C6 at around 820°C. The MC carbides remain stable down to about 680°C, where they dissolve in favor of the intermetallic compounds Ni3Ta (Struktur-bericht designation D0a) or Ni3Ti (Strukturbericht designation D024).
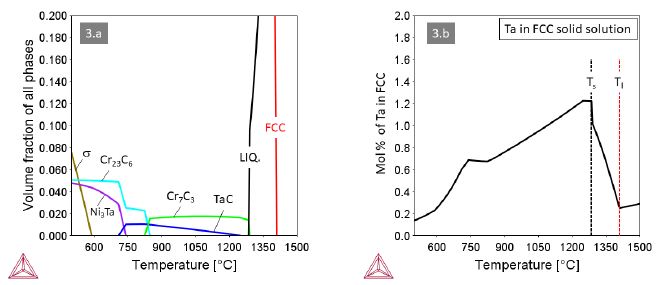
Figure 3: Thermodynamic equilibria in Alloy 3 with M=Ta
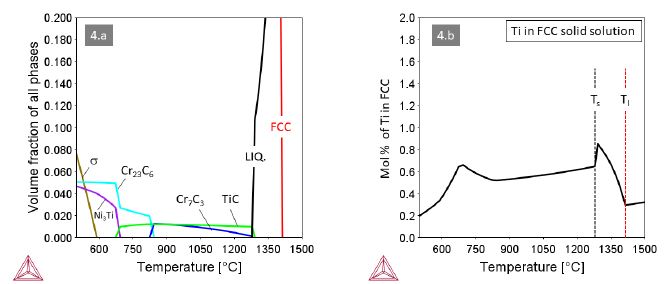
Figure 4: Thermodynamic equilibria in Alloy 4 with M=Ti
Comparing the alloys at case one must conclude that HfC is outstandingly stable from a thermodynamic viewpoint. In the absence of “parasitic” Cr7C3 carbides the Cr content of the FCC solid solution phase is also highest in Alloy 1, which may be relevant for the oxidation behavior. For convenience Table 2 lists the full composition of the FCC solid solution phase at T=1050°C, seen as a potential operation temperature.
Table 2: Calculated composition of the FCC solid solution matrix at T=1050°C
|
FCC composition |
Ni |
Co |
Cr |
C |
M = (Hf, Ta, W, Ti) |
| Alloy 1 (Hf), wt.% |
37.4 |
33.3 |
29.0 |
0.02 |
0.31 Hf |
| Alloy 2 (W), wt.% |
36.8 |
32.7 |
26.7 |
0.05 |
3.83 W |
| Alloy 3 (Ta), wt.% |
36.9 |
32.8 |
27.3 |
0.05 |
2.96 Ta |
| Alloy 4 (Ti), wt.% |
37.6 |
33.4 |
28.5 |
0.05 |
0.48 Ti |
Summary and Outlook
We revisited the formation of MC carbides in a model Ni-Co-Cr alloy containing carbon and the transition metal elements M being Hf, W, Ta, or Ti. A generic alloy composition was devised with carbon C and the transition metal M in nearly equimolar quantity, i.e. 1.15 at.% C and 1.20 at.% M. We used thermodynamic computations to reveal phase equilibria as function of temperature, thus helping to understand the sequence of phase formation and possible phase transitions. The results are summarized as follows:
(1) All alloys give rise to in-situ carbide formation, with distinct carbides solidifying from the last fraction of liquid in a so-called terminal eutectic reaction.
(2) Among the investigated transition metal elements M, hafnium is the only element to provide fully stable MC-type carbides and thus an alloy composed of FCC and HfC over the widest temperature range.
(3) For the nearly equimolar carbon and hafnium content, virtually all C and Hf are bonded in the HfC, leading to an FCC solid solution with low Hf and C content. In the absence of “parasitic” Cr7C3 the Cr-content of the FCC solid solution is highest.
On this thermodynamic background, one may further proceed to design alloys for casting and laser-based additive manufacturing. Laser based processing, e.g. laser powder bed fusion, opens up new pathways for controlling the size and distribution of in-situ formed carbides, taking advantage of the fast and directional solidification inside travelling melt pools. First experiments with an alloy composition close to Alloy 1 were already performed by the authors and submitted to publication.
Acknowledgement
The authors would like to acknowledge P. Berthod and co-workers from the Université de Lorraine, Institut Jean Lamour, France for many fruitful discussions on carbide-reinforced alloys.
Competing Interest Statement
The authors have no competing interests to declare.
Data Availability Statement
To reproduce our figures the Software Thermo-Calc and the database TCNi8 are required.
References
- Nakamura K, Yashima M (2008) Crystal structure of NaCl-type transition metal monocarbides MC (M=V, Ti, Nb, Ta, Hf, Zr), a neutron powder diffraction study, Materials Science and Engineering B, 148: 69-72.
- Murata Y, Suga K, Yukawa N (1986) Effect of transition elements on the properties of MC carbides in IN-100 nickel-based superalloy, Journal of Materials Science 21: 3653-3660.
- Berthod P (2009) High temperature properties of several chromium-containing Co-based alloys reinforced by different types of MC carbides (M = Ta, Nb, Hf and/or Zr), Journal of Alloys and Compounds 481: 746-754.
- Conrath E, Berthod P (2018) Properties of a HfC-reinforced nickel-based superalloy in creep and oxidation at 1100 C, Materials Science 53: 861-867.
- Wang R, Zhu G, Yang C, Zhou W, Wang D, et al. (2020) Novel selective laser melting processed in-situ TiC particle-reinforced Ni matrix composite with excellent processability and mechanical properties, Materials Science and Engineering A 797: 140145.
- Xia T, Wang R, Bi Z, Wang R, Zhang P.et al. (2021) Microstructure and mechanical properties of carbides reinforced nickel matrix alloy prepared by selective laser melting, Materials 14: 4792.
- Kim YK, Yu JH, Kim HS, Lee KA (2021) In-situ carbide-reinforced CoCrFeMnNi high-entropy alloy matrix nanocomposites manufactured by selective laser melting: Carbon content effects on microstructure, mechanical properties, and deformation mechanism, Composites Part B. Engineering 210: 108638.
- Chen H, Lu T, Wang Y, Liu Y, Shi T, et al. (2022) Laser additive manufacturing of nano-TiC particles reinforced CoCrFeMnNi high-entropy alloy matrix composites with high strength and ductility, Materials Science and Engineering A 833: 142512.
- Kaplan B, Blomqvist A, Selleby M, Norgren S (2015) Thermodynamic analysis of the W–Co–Cr system supported by ab initio calculations and verified with quaternary data, Calphad 50: 59-67.