DOI: 10.31038/CST.2018332
Abstract
Background: In 2017 we described 5-year histological and disease-free survival data from a series of 429 patients with cancers of the uterine corpus (UCC), observing similar percentages to those described in the medical literature. The only difference we found with other published studies was a slightly lower percentage of serous carcinomas (SC) in the Western countries, but similar to the 3% of all SC in Japan [1]. The objective of this study was to evaluate the prognosis of uterine corpus cancer and the influence of tamoxifen on the histological types of uterine body cancer, and their long-term survival (20 year follow-up).
Methods: Two series of patients were included in this study: [1] 429 patients with uterine corpus cancer and [2] 1385 patients with breast cancer. The second group included 1057 patients who had been treated with tamoxifen and 328 who had not been treated. All women had been diagnosed at the same Hospital (General University Hospital of Vigo). Crossing both series, we observed that 51 women were diagnosed with both uterine and breast neoplasias.
Previously, we had excluded those cases of breast cancer (n = 1051) at the time of closing the data collection as we were not certain whether or not they had received hormonal treatment. We compared histological types of corpus uterine cancer for women with and without a prior diagnosis of breast cancer. Age of diagnosis, uterine tumor stage, histological cell type, histological grade, treatment and time 20 year free disease were analyzed using Kaplan-Meier and log-rank test analysis.
Results: Women diagnosed with UCC with a personal history of a previous breast cancer treated with tamoxifen had UCC with significantly more undifferentiated histological types than those with UCC who had not received tamoxifen: with a significantly higher proportion of uterine papillary serous carcinomas (3.2 % vs. 1.9 %), clear cell (6.4 % vs. 3.7 %), indifference carcinoma (3.2% vs. 0.8%) and sarcomas (9.7% vs. 3,7%) compared to those without a personal history of breast cancer (p < 0.001). There were no differences with regards FIGO Stage uterine corpus cancer with and without breast cancer history (p > 0.05). The 20-years free of disease survival in patients with endometrial adenocarcinoma was significantly decreased in women with tamoxifen breast cancer history (p = 0.04).
Conclusions: In this study, our data showed that women with uterine corpus cancer who had a personal history of breast cancer treated with tamoxifen had an increased risk of developing more aggressive UCCs and a lower 20-years free of disease survival. The odds ratio was 2.4. We think that a regular uterine endometrial and myometrial follow-up in the very long term is necessary in women diagnosed with breast cancer and treated with tamoxifen.
Keywords
breast cancer (BC), FIGO (International Federation of Gynecology and Obstetrics), endometrial adenocarcinoma, serous carcinoma (SC), tamoxifen, uterine corpus cancer (UCC), uterine papillary serous carcinoma (UPSC)
Introduction
Tamoxifen remains a first-line adjuvant treatment for premenopausal breast cancer patients with estrogen receptor-α (ERα) positive tumors, and is often prescribed to postmenopausal females with ERα+ tumors. Tamoxifen functions as an antagonist to ERα and blocks its signaling pathway in ERα+ breast cancer cells. Tamoxifen has been prescribed to millions of females for breast cancer prevention and/or treatment. However, tamoxifen is also known to significantly enhance the risk of developing endometrial lesions, including hyperplasia, polyps, carcinomas, and sarcoma [2].
Previous case-controlled studies have shown that tamoxifen has allowed for an increase in the survival of women with breast cancer, reducing the risk of relapse after 5 years of treatment, but it was potentially carcinogenic to the endometrial tissue [3–4]. In one of the largest population studies done by Chen et al. In 2013, on 74,280 breast cancer patients, the use of tamoxifen for more than three years or in patients older than 35 years of age was associated with a significant increased risk for developing endometrial cancer, with odds ratios of 2.94 and 4.08 respectively [5].
Breast cancer treatment with tamoxifen and its association with a higher risk for uterine corpus cancer has been recognized for many years now, but its long-term effect has not been clearly recognized nor monitored. Some studies have found a small incremental increase in the risk ratio for endometrial carcinoma in postmenopausal breast cancer patients treated with tamoxifen over 5 years [5,6], while other studies have found that the risk ratio for developing endometrial carcinoma while on tamoxifen is similar to that when using other type of estrogens [7].
A few studies have associated the use of tamoxifen with the development of low level tumors [8,9] that in the majority of the cases are state I FIGO, low level and histological subtypes similar to those detected in women non using tamoxifen [10]. However, an increasing number of studies have found that women who received tamoxifen for breast cancer, have an increased risk of more aggressive endometrial cancers, with more non-endometrioid types, in more advanced stages [3,4] and with worse prognosis [11].
According to Hoogendoorn et al. [12], tamoxifen-associated tumors have less favorable histological features and are more agresive and with a worse survival rate after 3 years (82% versus 93% p = 0.0001). These tumors in long-term tamoxifen users were also more often steroid receptor-negative. Previously, Bergman et al. [13] had associated the long-term use of tamoxifen (2–5 years) to a higher incidence of malignent mixed mesodermic tumors (carcinomas) or sarcomas of the endometrium (15.4% versus 2.9% p≥0.02). Similar results have been published by Narod [11] and Lasset [14].
These conflicting reports in the literature may be a result of heterogeneous cohorts and an insufficient long-term follow-up with the patients. The objective of our study was to analyze the prognostic of the uterine corpus cancers and the influence of tamoxifen on the histological types of these types of cancers and patients’ long-term survival.
Materials And Methods
An observational prospective-retrospective and longitudinal study was conducted at General University Hospital in Vigo (Spain), in patients diagnosed with uterine corpus cancer (UCC) and breast cancer (BC) from November 1984 through September 2010. A total of 1814 patients were included in this study and in-situ breast cancers were not included. The tamoxifen minimum use required for this study was 3 years. The age of diagnosis, uterine tumor stage (FIGO, Staging 2009), histological type, tamoxifen treatment and time free disease were recorded. To analyze this study cohort and determine the 20-year disease-free specific survival, Chi-squared test and Kaplan-Meier analysis with log-rank test were performed. All statistical analysis were made using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, Illinois, USA) version 22.0 for Windows.
Results
Crossing two series of patients diagnosed with UCC (n = 429) and another with BC (n = 1385) we observed that fifty-one women (n = 51) were diagnosed with both neoplasias, uterine and breast (Table 1). Our data showed that two thirds of these patients were diagnosed with UCC post breast cancer diagnosis, mostly associated with a previous treatment with tamoxifen.
Table 1. Patients (n = 51) diagnosed with both cancers: uterine corpus (UCC) and breast (BC)
|
UCC before breast cancer |
UCC synchronous to breast cancer |
UCC after breast cancer |
|
|
15 (29.4 %) |
1 (1.9 %) |
35 (68.6 %) |
|
|
NO tamoxifen |
no tamoxifen |
tamoxifen treatment |
|
|
4 (11.4 %) |
31 (88.6 %) |
||
In our study we analyzed 1385 breast cancer cases, with 76.3% of these patients (n = 1057) receiving tamoxifen treatment and 23.7% (n=328) not receiving it. Our data indicated that 31 of the 1057 patients (2.9%) developed UCC post-Tamoxifen treatment, whereas only 4 of the 328 patients (1.2%) developed UCC without treatment with tamoxifen. Therefore, we found an unusual ratio for breast cancer patients treated with tamoxifen and uterine corpus cancer of 2.4 (p = 0.05)
When we studied the different histological types and uterine tumor stages by FIGO 2009, the patients were classified into three groups: (a) 51 patients with both types of cancers (uterine and breast cancer), (b) 31 patients with uterine corpus cancer after treatment with tamoxifen for breast cancer and (c) 372 patients diagnosed with uterine corpus cancer only, no breast cancer (Table 2). Our data indicated that groups a and b had a significantly higher proportion of uterine papillary serous carcinomas (UPSC), clear cell (CC), indifference carcinoma and sarcomas compared to uterine cancer only (5.9% and 3.2 % vs. 1.9% for UPSC, 5.9% and 6.4 % vs. 3.7 for CC, 1.9% and 3.2 % vs. 0.8% for indifferent and 13.7% and 9.7 % vs. 3.7% for sarcomas; p < 0.001). However, there were not differences with regards to the FIGO Stage uterine corpus cancer with and without breast cancer (p > 0,05)
Table 2. Histological types of Uterine corpus cancer with and without breast cancer. Uterine-only breast with tamoxifen were included inside the Uterine-breast group for the counting on total cases ( 51 + 372 = 423)
|
Histology |
Uterine-breast a (n = 51) |
Uterine- only breast with Tamoxifen b (n = 31) |
Uterine only c (n = 372) |
Total (n= 423) |
|
Endometrioid |
33 (64,7%) |
23 (74,2 %) |
321 (86,3%) |
354 |
|
UPSC |
3 (5,9%) |
1 (3,2 %) |
7 (1,9%) |
10 |
|
Clear cell |
3 (5,9%) |
2 (6,4 %) |
14 (3,7%) |
17 |
|
Indifferent |
1 (1,9%) |
1 (3,2 %) |
3 (0,8%) |
4 |
|
Sarcoma |
7 (13,7%) |
3 (9,7 %) |
14 (3,7%) |
21 |
|
Others |
4 (7,8%) |
1 (3,2 %) |
13 (3,5%) |
17 |
|
Total |
100% |
100% |
100% |
p < 0,001 |
Finally, to evaluate whether the use of tamixofen could influence the prognosis of UCC at long term, we followed a 20 year free disease survival of 383 women with UCC only, and we compared it with the same time period for the 31 women diagnosed with UCC after being treated with tamixofen for breast cancer (Figure 1). Our data clearly indicated that the cumulative survival rate was significatively lower in women with uterine corpus cancer after breast cancer treated with tamoxifen, with a decrease of survival of 20% after 2 years and 40% after 12 years, compared with a slower pace 20% decrease in women with UCC only after 20 years.
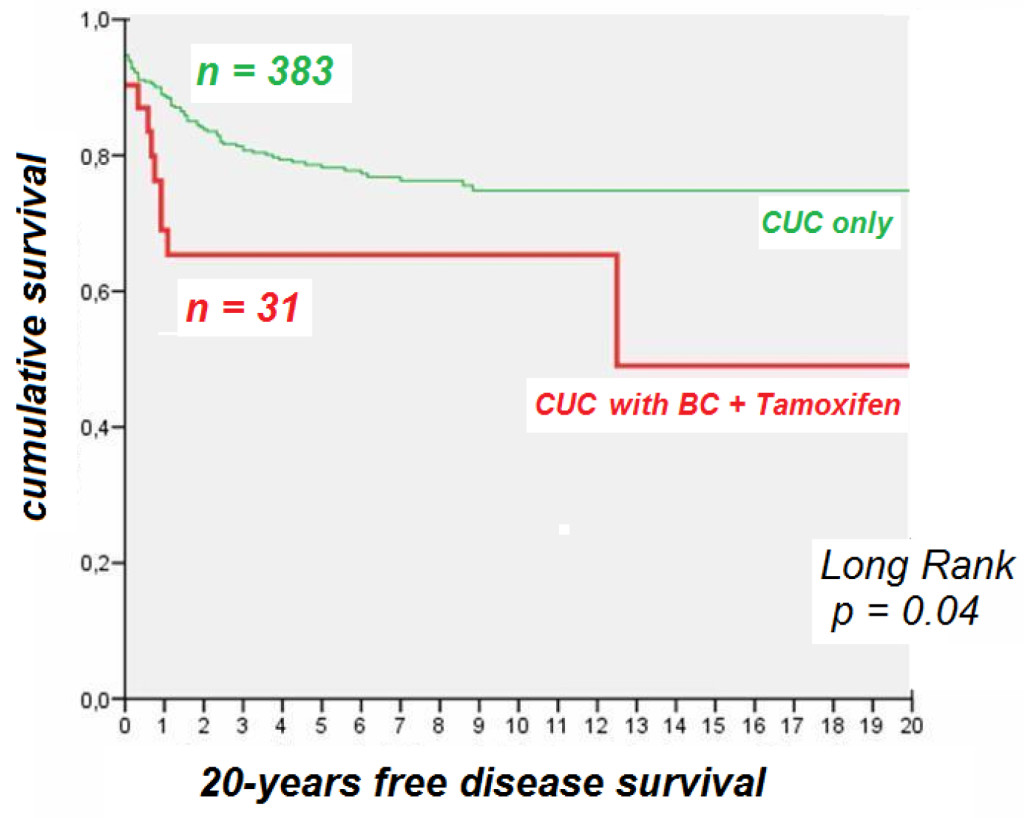
Figure 1. The 20-years disease free survival in patients with uterine corpus cancer (UCC) and UCC and breast cancer (BC) treated with tamoxifen.
Discussion
Breast, ovarian, and uterine corpus cancers are common female cancers and categorized as hormone-related diseases. The recent population-based study carried by Chen et al. in Taiwan to test the hypothesis of bidirectional associations among these cancers found similar results to our study [15]. Using a cohort of 110,112 cases with primary female cancers including uterine corpus cancer (11,146 cases), ovarian cancer (12,139 cases), or breast cancer (86,827 cases) from the Taiwan Cancer Registry from 1979 to 2008, the pairwise risks of second cancer among uterine corpus, ovary, and breast cancer cases were evaluated. A reciprocal relationship was found for these three female cancers, particularly most prominent between uterine and ovarian cancers, followed by breast and uterine cancers as well as breast and ovarian cancers. The overall risk of second cancers was highest within the first 5 years after the diagnosis of primary cancer. The bidirectional relationships suggest common risk factors among these three female cancers.
Our data showed that women with breast cancer and tamoxifen use had a relative risk of 2.4 to develop UCC when compared with women who didn’t use it. We also found that women with prior history of breast cancer and treated with tamoxifen were more likely to be diagnosed with a uterine corpus cancer of high risk (high histologic grade and no endometrioid types) as compared to those with uterine corpus cancer without prior breast cancer. One possible explanation for this difference could be the existence of a genetic association between breast and papillary serous cancers, as described by Horneich et al. in 1999 [16] but an association to the use of tamoxifen is a very plausible alternative. Our study found a significantly higher number (88.6%) of uterine corpus cancers developed in breast cancer patients after treatment with tamoxifen compared to the lower number (11.4%) of uterine corpus cancers developed in women who have had breast cancer without tamoxifen treatment. This difference could be due to the hormone-dependent characteristics linked to breask cancer and uterine cancer, as found previously in other studies [2,5] or the oncogenic effect of tamoxifen [17].
Bernstein at al in 1999 also found that the risk of endometrial cancer increased greatily in women with more than 5 years exposure to tamoxifen when compared to less than 5 years and nonusers [18]. In our study we found that in patients treated with tamoxifen, 15 endometrial adenocarcinomas appeared during the frst 5 years and 15 more tumors during the next 15 years, whereas carcinosarcomas were not detected during the first 5 years, and only 2 tumors appeared during the next 15 years. Only one case of leiomiosarcoma was detected after 6 years of breast cancer diagnosis (data not shown). It is important to highlight that when we evaluated the endometrial adenocarcinomes for histological subtypes, we observed the most aggresive types (serous and clear cell) in the 3 cases of women who have been treated with tamoxifen for breast cancer (Table 2)
Chronologically, the relative risk to develop an endometrial cancer is higher at two points: between the second and fifth year and between the 9th and 10th year after a breast cancer diagnosis. Therefore these data suggest that women with breast cancer treated with tamoxifen should be monitored for longer periods of time due to their higher risk to develop more aggresive types of uterine corpus cancer many years after ending their treatment, although these cancers could also be due to their association with increased age.
If we evaluate a 20-years disease free survival instead of a 3–5 year period, considering that the tumors can develop several years after ending the treatment with tamoxifen, our data support the fact that the survival rate at those two time periods is significatively different, decreasing overtime for women who underwent this treatment
(Figure 1). By continuing to studying the long term survival at 20 years, we were able to evaluate more precisely the long-term effect of tamoxifen, as already suggested in other studies [5, 15, 19–22]. Based on these data, we believe that it is neccesary to create an increased awareness among gynecological health providers. Regular follow-ups, pelvic examinations and uterine endometrial screening may play a very important role for endometrial carcinoma early diagnosis in women who have undergone treatment with Tamoxifen for breast cancer, and it might decrease the risk incident for uterine cancer in the long term.
Our study shows that women with uterine corpus cancer (UCC) who have had a personal history of breast cancer treated with tamoxifen, have an increased risk of developing more aggressive UCC overtime, and a lower 20-years free disease survival rate. Therefore We highly suggest that a specific uterine endometrial and myometrial follow-up in the very long term is necessary and will help to improve the lives of women diagnosed with breast cancer and treated with tamoxifen.
References
- Cameselle-Teijeiro JF, Valdés-Pons J, Cameselle-Cortizo L, Fernández-Pérez I, Lamas González MJ, et al. (2017) Tumours of the Uterine Corpus: A Histopathological and Prognostic Evaluation Preliminary of 429 Patients. J Clin Med Exp Images 1: 11–19.
- Hu R, Hilakivi-Clarke L, Clarke R (2015) Molecular mechanisms of tamoxifen-associated endometrial cancer (Review). Oncology Letters 1495–1501.
- van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrere CH, Otter R, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet 343: 448–452.
- Swerdlow AJ, Jones ME; British Tamoxifen Second Cancer Study Group (2005) Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst 97: 375–384. [crossref]
- Chen JY, Kuo SJ, Liaw YP, Avital I, Stojadinovic A, et al. (2014) Endometrial Cancer Incidence in Breast Cancer Patients Correlating with Age and Duration of Tamoxifen Use: a Population Based Study. Journal of Cancer 5: 151–155.
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, et al. (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295: 2727–2741. [crossref]
- Eltabbakh GH, Mount SL (2001) Tamoxifen and the female reproductive tract. Expert Opin Pharmacother 2: 1399–1413. [crossref]
- Neven P, De Mylder X, van Belle Y, van-Hooff I, Vanderick G (1998) Longitudinal hysteroscopic follow-up during tamoxifen treatment. Lancet 351: 36.
- Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F (2000) Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol 78: 181–186.
- Peters-Ingl C, Frank W, Danmayr E, Frield HP, Leodolter S Medl M (1999) Association between endometrial cancer and tamoxifen treatment of breast cancer. Breast Cancer Res Treat 54: 255–260.
- Narod SA, Pal T, Graham T, Mitchell M, Fyles A (2001) Tamoxifen and risk of endometrial cancer. Lancet 357: 65–66. [crossref]
- Hoogendoorn WE, Hollema H, van Boven HH, Bergman E, de Leeuw-Mantel G, Platteel I, et al. Comprehensive Cancer Centers TAMARISK-group: Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res Treat 112: 99–108.
- Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, Van Leeuwen FE (2000) Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 356: 881–887.
- Lasset C, Bonadona V, Mignotte H, Brémond A (2001) Tamoxifen and risk of endometrial cancer. Lancet 357: 66–67. [crossref]
- Chen MC, Lee KD, Lu CH, Wang TY, Huang SH, Chem CY (2018) Bidirectional association among female hormone-related cancers: breast, ovary and utery corpus. Cancer Medicine 2018 (Apr): 1–8.
- Hornreich G, Beller U, Lavie O, Renbaum P, Cohen Y, Levy-Lahad E (1999) Is uterine serous papillary carcinoma a BRCA1-related disease? Case report and review of the literature. Gynecol Oncol 75: 300–304.
- Burke TW, Tortolero-Luna G, Malpica A, Baker W, Whittaker L, Johnson E, et al. Endometrial hyperplasia and endometrial cancer. Obstet Gynecol CIin North Am 1996; 23: 411–456.
- Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney, et al. (1999) Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst 91: 1654–1662.
- Valdes-Pons J (2011) Breast cancer endometrial pathology (doctoral thesis). Santiago de Compostela: Santiago de Compostela University ISBN 978–84–9887–726–7.
- Spanos WJ Jr, Peters LJ, Oswald MJ (1986) Patterns of recurrence in malignant mixed müllerian tumor of the uterus. Cancer 57: 155–159. [crossref]
- Pierce SR, Stine JE, Gehrig PA, Havrilesky LJ, Secord AA, et al. (2017) Prior breast cancer and tamoxifen exposure does not influence outcomes in women with uterine papillary serous carcinoma. Gynecologic Oncology 144: 53 -535.
- Varras M, Polyzos D, Akrivis Ch (2003) Effects of tamoxifen on the human female genital tract: review of the literature. Eur J Gynaecol Oncol 24: 258–268. [crossref]