DOI: 10.31038/CST.2019413
Abstract
Purpose: To determine the frequency of albino skin cancers and to describe the difficulties related to the diagnostic and therapeutic management of these patients in Guinea.
Material and methods: This was a retrospective cohort study on albinos attending Surgical Oncology Unit of Donka National Hospital for skin cancer from March 17, 2007, to December 17, 2016.
Results: We identified the 30 albinoes who presented 41 skin cancer lesions. There were 18 (60.0%) women and 12 (40.0%) men. The average consultation delay was 28.3 months. Patients were housewives in 10 cases (33%), merchants in 8 cases (26.6%) and students in 6 cases (20.0%). The primary sites were the face in 22 cases (73.3%), trunk in 4 cases (13.3%) and neck in 3 cases (10.0%). There were squamous cell carcinoma in 29 cases (96.7%) and sarcoma 1 case (3.3%). The clinical stage was localized in 16 cases (53, 3%), locally advanced in 13 cases (43.3%) and metastatic in 1 case (3.3%). Wide surgical excision performed in 17 (56.7%) for 28 lesions. Wound closure was achieved by a myocutaneous flap in 15 cases, directed scarring in 7 cases, direct suture in 4 cases and skin graft in 2 cases. After a median follow-up of 8 months, 2 patients presented with relapse and 3 new tumor lesions and 9 (30.0%) died. At 24 months, overall survival was 29.0%.
Conclusion: The incidence of skin cancer is high among albinos. Late diagnosis and inaccessibility to means of treatment are factors limiting the management of this vulnerable group.
Keywords
skin cancers, albinos, squamous cell carcinomas, late diagnosis
Introduction
Albinism is an inherited genetic disorder characterized by hypopigmentation of the skin, hair and / or eyes due to a lack or reduced cutaneous production of melanin [1]. Albinos are very sensitive to sunlight damage to the skin [2]. There are two main types of albinism, oculocutaneous (eyes, skin, and hair) and ocular. Skin cancer is a major cause of morbidity and mortality in albinos. They develop precancerous and cancerous lesions at a young age and suffer from advanced skin cancers in the third and fourth decade of their life [3].
It is clear that there are social discrimination and stigma directed towards albinos. As a result of this social discrimination, they are severely limited in the search for medical care when they have significant deformities following advanced disease [4]. Few data exist on skin cancer in albinos in Guinea. The previous study in our unit showed that skin cancers accounted for 7.8% of cancers and 4 of the 84 cases were albinos [5].
These patients have very little access to prevention and care whereas they should be under dermatological screening. Surgical treatment is the only means of treatment available for healing in our settings. This study aimed to determine the frequency and discuss the management of skin cancers of this vulnerable population at Surgical Oncology Unit of Donka National Hospital.
Material and Methods
Settings
Data collection took place at the Surgical Oncology Unit (SOU) of Donka National Hospital, Conakry University Hospital. This unit, created since 2007, receives albino patients from the dermatology and other departments for the skin cancers treatment.
Study design
This was a retrospective cohort study of albino’s patients with cutaneous cancers at the SOU from May 17, 2007, to December 17, 2016. The consultation records, albino patient records, pathology reports, and the operating protocol register completed data collection forms.
Population and data collection
We included records of albino patients who had histologically confirmed skin cancers among other cases of skin cancer during the study period. Records of albino patients with histologically unconfirmed skin tumors or other types of cancer were excluded.
The socio-demographic data studied included age, sex, ethnicity, occupation, and residence. The history of skin cancer, actinic lesions, smoking, and alcohol consumption was recorded. Infection with the human immunodeficiency virus (HIV) was sought by retroviral serology.
Consultation delay from the moment that the patient observed the lesion and came for consultation was identified. Iterative excision was defined by this done before the patient came to the SOU. Clinical data included clinical appearance, tumor size in centimeters, number of lesions, and primary site.
Histological confirmation was obtained on an incisional biopsy specimen or on a complete excision sample. Histological types were classified according to the World Health Organization (WHO) [6]. Adjacent infiltration of muscles or bone was reported. Regional lymphadenopathies were checked by clinical assessment. The sparing assessment was completed by chest x-ray (looking for pleuropulmonary opacities) and abdominal and pelvic ultrasound (looking for hepatic hypoechoic images). The TNM clinical classification of the International Union against Cancer (UICC) 2010 [7] was used to classify skin cancers in this study.
The types of surgical excision, closure of the operative wound, excision margin status, operative complications, and hospitalization were described. Some patients benefited from palliative chemotherapy (5- Fluoro-uracil and cisplatin). There is no radiotherapy in our country. Follow-up time, rates of recurrence and appearance of new tumor foci, and survival were assessed.
Data analysis
Statistical data were analyzed with statistical package for the social sciences (version 21.0 for Windows, SPSS, Inc., Chicago, IL). Categorical variables were shown as the frequency and percentage (%), and continuous variables were presented as the mean (± standard deviation) and / or median and interquartile range (IQR). Patients lost to follow-up were included in the survival analysis. Factors associated with survival were studied in relation to surgical excision and clinical stage. Survival was calculated according to the Kaplan Meier method. The log-rank test was used to analyze prognostic factors. The cox model was used to search for independent prognostic factors. The test was significant if the p-value was less than 0.05.
Ethical considerations
The data analyzed in this study respected anonymous and confidential principles.
Results
From 2007 to 2016, we recorded 41 albinos with clinically suspected skin cancers. Eleven cases were excluded because of the absence of histological examination. We included the records of 30 patients who had histologically confirmed skin cancer. They accounted for 21.9% of 137 patients with skin cancer at the Donka SOU during the study period. Figure 1 shows the number of cases per year.
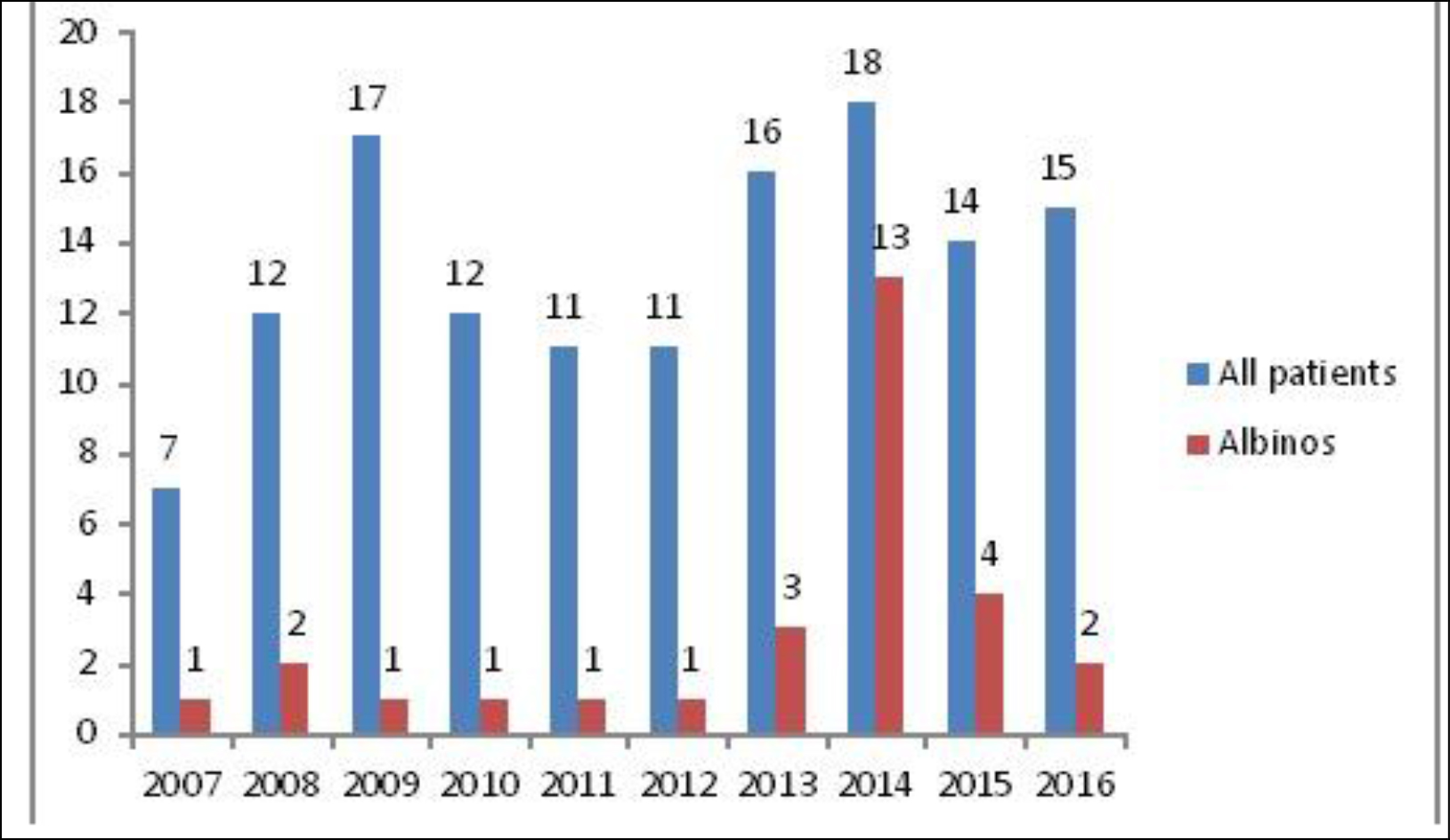
Figure 1. Number per year of albinos among patients presenting skin cancer
Sociodemographic Data
The age ranged from 17 to 76 years with an average of 31.6 years (± 14.0) and a median of 31.5 years (IQR 21.7–45.2). Patients aged 30 and under accounted for 50%. There were 12 men (40.0%) and 18 women (60.0%). Patients were housewives in 10 cases (33.3%), merchants or traders in 7 cases (23.3%) and students in 6 cases (20.0%). The patients were from Conakry region in 8 cases (30.0%), Kindia in 7 cases (23.3%) and Kankan in 6 cases (20.0%). Fulani accounted for 14 cases (46.7%), Mandingo 11 cases (36.7%), Soussous 4 cases (13.6%) and Kissi 1 cases (3.3%).
Risk factors
A patient has been previously treated for skin cancer. An actinic lesion has been found in one patient. Smoking and alcohol consumption were reported in the lifestyle of 5 (16.7%) and 3 (10.0%) patients, respectively. HIV infection was found in one patient.
Clinicopathological features
The median consultation delay was 12 months (IQR 8.5–46.0). Before admission, iterative excision was noted in 3 patients. For the 30 patients, there were 41 cancerous skin lesions or 1.4 lesions per patient. Patients had one lesion in 23 cases (76.7%), 2 lesions in 4 cases (13.3%), 3 lesions in 2 cases (6.7%) and 4 lesions in 1 case (3.3%).
The primary site was the face in 22 cases (73.3%), the trunk in 4 cases (13.3%) and the neck in 3 cases (10.0%).
The clinical aspect was nodular in one patient and ulcero-budding in 29 patients. The tumor size ranged from 1–47cm with a median of 5cm (IQR 3.0–12.0). The tumor infiltrated adjacent muscles in 6 cases (20.0%) and bone in 1 case (3.3%). There was regional lymph node involvement in 7 cases (23.3%) and hepatic metastases in one patient. The clinical stage, according to TNM classification of UICC 2010, was localized in 16 cases (53, 3%), locally advanced in 13 cases (43.3%) and metastatic in 1 case (3.3%). Table 1 presents sociodemographic data, the primary sites, and clinical tumor (T) classification.
Table 1. Sociodemographic data, Primary sites and clinical primary tumor (T) classification (UICC 2010) of skin cancer in albinos (n = 41)
|
Characteristics |
Number |
% |
|
Age – 10–19 – 20–29 – 30–39 – 40–49 – 50–59 – 60 & more |
5 8 8 6 2 1 |
16.6 26.6 26.6 20.0 6.6 3.3 |
|
Occupations – Housewives – Pupils / Students – Merchants or sellers – Drivers – Artist – Teacher – Veterinarian – None |
10 6 8 2 1 1 1 1 |
33.3 20.0 26.6 6.6 3.3 3.3 3.3 3.3 |
|
Residence – Conakry – Kindia – Kankan – Faranah – Boké – Mamou – Labé |
9 7 6 3 2 2 1 |
30.0 23.3 20.0 10.0 6.6 6.6 3.3 |
|
Primary sites – Face – Trunk – Neck – Arms – Eyelids – Multiple |
22 4 3 1 1 1 |
73.3 13.3 10.0 3.3 3.3 3.3 |
|
Primary tumor (T) classification – T1 – T2 – T3 – T4 – Tx |
4 6 13 2 5 |
13.3 20.0 43.3 6.7 16.7 |
The diagnosis based on the histological examination of the biopsy specimen in 20 patients (66.7%) and the excisional specimen in 10 (33.3%) patients. This was squamous cell carcinoma in 29 cases (96.7%) and sarcoma 1 case (3.3%).
Treatment
A wedge surgical excision was performed in 17 patients (56.7%) for 28 lesions. Radical cervical lymph node dissection was performed in the second time in one patient. Wound closure was obtained by a simple suture in 4 cases, cutaneous graft in 2 cases, myocutaneous flaps in 15 cases and directed scarring in 7 cases. Excision margins were free in 7 documented cases. Infection of the operative site (1 case) and delay of healing (1 case) were complications observed. This intervention required hospitalization in 7 cases. The median hospital stay was 6.5 days (IQR 4.5–20.7). Salvage and palliative chemotherapy were performed in 2 and 1 patients respectively. The clinical response to this chemotherapy was disease progression in all cases.
Follow-up
Nine patients were seen once at the consultation. After a median follow-up time of 8 months [95% CI 2.3–13.7], we observed a relapse in 2cases (6.7%), the appearance of new tumor lesions in 3 (10%) patients and 9 (30%) patients had died. Figure 2 shows the old scars and new tumor lesions.
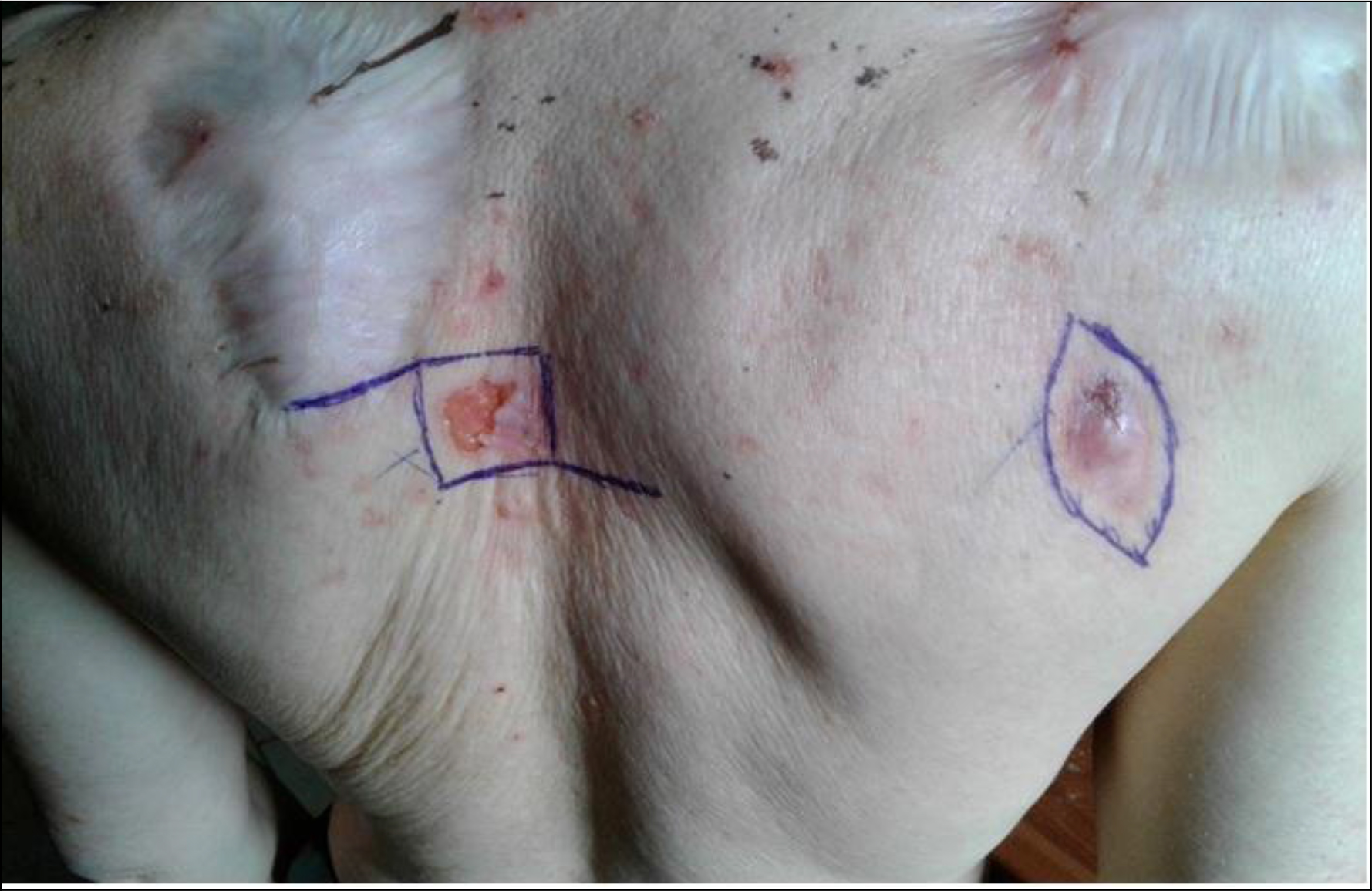
Figure 2. Old scars and new tumor lesions
Overall survival after a median follow-up of 8 months was 70.0%. At 24 months, overall survival was 29.0% (Figure 3). This survival was correlated with being operated upon (88.2% versus 46.2%) (p = 0.02). It varied according to the stage: 87.5% for the localized stage, 53.8% for the locally advanced stage and none for the metastatic stage (p = 0.056). None of the factors were independent after analysis by the Cox model.
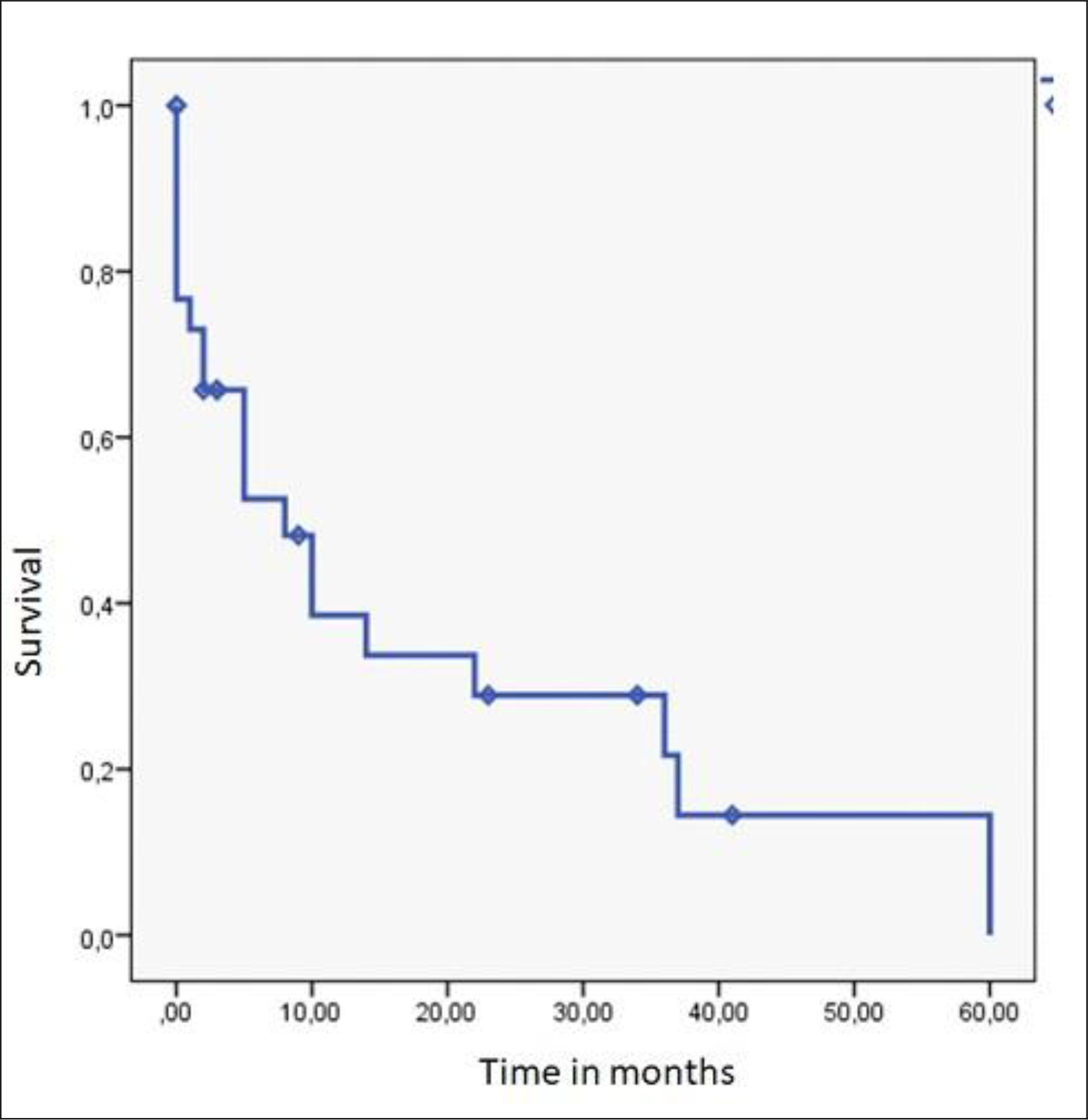
Figure 3. Overall Survival of skin cancers in Albinos (n = 30)
Discussion
The limitations of this study were the low number of histologically confirmed cases of skin cancer in albinos. Despite this, we showed the high frequency of skin cancer in albinos, which accounts for about one-fifth of all skin cancers in this study. This frequency was similar according to Kromberg et al [8] in South Africa. It was higher than that of Mabula et al [1] in Tanzania which was 13.2%. In an Imo State plastic surgery department in Nigeria, skin cancer in albinos represented 67.0% [9].
As in other findings [1,3,10], the patients are in their third decade.
The high frequency in the Conakry region and surrounding areas is related to the proximity of the reference services (Dermatology and Oncological Surgery Unit) in the management of skin cancers.
The socio-professional groups involved in this study were those (housewives, tradesmen, students) who were exposed to the sun. Patients working outdoors, under the sun accounted for 75.0% according to Opara and Jiburum [9]. The use of wide-brimmed hats, long-sleeved shirts, scarves on the neck, sunglasses, and sunscreens could reduce the occurrence of cancer in these areas of the body.
Smoking found in some cases would increase the risk of CCS [11]. Actinic lesions was reported only in one patient where is found by Emadi et al [12]in 37.7% of women and 29.1% of men.
HIV infection, diagnosed in a patient, would increase the frequency of cutaneous cancers in albino but the etiopathogenic mechanism is not known [13]. HIV infection was reported in 6.2% of albino patients in the Mabula et al [1] series.
Clinicopathological features
The long delay, varying from 3 to 120 months, was reported by Opara and Kiprono [3,9]. This late presentation was mainly related to poverty and ignorance. Treatment in peripheral hospitals and among traditional healers is mentioned in some studies [1]. Three of them were poor first management in peripheral health facilities, hence the need to improve continuing health professionals training on early detection. The multiple lesions were common in the same patient. The mean number of outbreaks was 1.4 per patient in our study and 1.9 lesions per patient according to Opara [9]. The part of the body (face, trunk, and neck) exposed to the sun, were the most common primary sites. Although none exist in our study, rare cases of localization on the genitals and the lower limbs have been described by Mabula et al [1].
The average size of 5cm is reported in other studies [1, 14].
The diagnosis of cutaneous cancer was confirmed on the histological analysis of the biopsy specimen in 2/3 of the cases and the operative specimen 1/3 of the cases. In the first case, it is often advanced lesions, multiple or from other services with the results. In the second case, these are patients who have resectable lesions, who accept the surgical treatment after assessment; the resection is then both diagnostic and therapeutic.
Almost cases were squamous cell carcinoma in this study. This was reported by several studies [1,3,8,15]. There are no basal cell carcinomas and melanomas, but these forms reported [1,3,9]. In contrast, cutaneous sarcomas in albino are very rare; only one case in this study. Rare cases of sarcomas have been reported [16,17].
Adjacent muscle or bone, and cervical lymph node involvement indicate advanced stages in these patients. These lymphadenopathies were all related to SCC. Mabula et al [1] reported 12.5% of regional lymphadenopathies in 64 patients. In this study, liver metastases were present in one patient whereas Mabula et al [1] described liver, lung, bone and nerve metastases in 6 out of 64 patients.
Treatment
One of the limitations of this study was the small number of patients treated. Our 60% of patients treated were greater than those of a previous study in which 46 of 102 skin cancer patients were treated. [18]. This difference can be explained by the effect of the campaign of early detection and management of albinos in 2014. However, in Africa, black albinos often suffer social discrimination because of superstitious beliefs and the stigma associated with albinism. They are often rejected by their communities, with a resulting delay in the search and treatment of any precancerous or malignant keratosis lesions. Thus, at the time of diagnosis, the lesion is often advanced and has a poor prognosis [13].
Surgery has been reported as the mainstay of treatment for the majority of skin cancers in albinos [19,20]. Wide excision is the most important to prevent local recurrence. Good results can be achieved with radical surgery and optimal surgical margins as well as the reconstructive procedure when needed. In the current study, wide local excision, with skin flap closure or graft was the most performed surgical procedure as in many studies [1,4,9].
One patient underwent cervical dissection for regional lymph node relapse, after treatment of the primary lesion by wide excision. Mabula et al [1] performed a single lymph node dissection among the 64 patients in their study. We did not perform systematic lymph node dissection because we considered that the lymph node would be related to secondary infection. In addition, all lymph nodes decreased after the excision of the primary lesion.
It is often an outpatient surgery. But hospitalization may be needed as for 7 patients who underwent extensive resection and / or to avoid complications while Mabula et al [1] hospitalized more than 90.0% of patients for their treatment. Wound Infection and delayed healing were surgical complications observed in one patient. In addition to these complications, flap necrosis and skin graft loss have been described [1].
Three (3) patients received cisplatin and 5 fluorouracil-based chemotherapy and results were progression disease. Although cases of complete or partial responses have been reported after chemotherapy with adriamycin and platinum salts (carboplatin or cisplatin) [21, 22], there is not enough data to confirm the role of neoadjuvant chemotherapy in locally advanced skin cancers in albinos.
In this study, we had problems to follow-up these patients. The median time to follow up was 8 months. These follow-up problems have been reported by other authors as well [1,9]. Despite these follow-up problems, we were able to assess the local control by the recurrence rate which was 2 out of 17 cases, representing a relapse rate of 11.7%. This rate seems less than that found by Mabula et al [1] which was 30.0%. These differences could be explained by surgical margins status. In this study, surgical margins vary 1–2cm as reported in the previous study on skin cancer surgery in our unit [18]. Our patients did not receive radiotherapy, which is indicated in cases of unresectable locally advanced cancers, in the adjuvant situation and in cases of relapse [1,9]. In addition, radiotherapy can optimize local control after surgical excision of the lesion.
In this study, we noticed the appearance of new tumor lesions in 3 patients. Thus, multiple lesions, relapse, and new tumor lesion mean that albinos can undergo multiple skin surgeries during their lifetime [14].
Approximately one-third of patients died in this study while the mortality was 4 out of 64 patients in Tanzania [1]. This high mortality is related to not being operated upon and has been higher in advanced cases.
Conclusion
The incidence of skin cancer is high among albinos. Late diagnosis and inaccessibility to means of treatment are factors limiting the management of this vulnerable group.
Acknowledgment
We said thanks to Mr. Thierno Boubacar Balde for the patient records availability
Ethics approval and consent to participate: In this retrospective study, data were collected anonymously and confidentially. Patients signed the consent form for the use of data contained in their records.
Consent for publication: Patients signed the consent form for the use of data contained in their records.
References
- Mabula Jb, Chalya Pl , Mabula Dm (2012) Skin cancers among albinos at a university teaching hospital in northwestern Tanzania: retrospective review of 64 cases. BMC Dermatology 12: 5.
- Ramalingam VS, Sinnakirouchenan R, Thappa MS (2009) Malignant transformation of actinic keratoses to squamous cell carcinoma in an albino. Indian J Dermatol 54: 46–48.
- Kiprono SK, Chaula BM, Beltraminelli H (2014) Histological review of skin cancers in African Albinos: a 10-year retrospective review. BMC Cancer 14: 157. [crossref]
- Ademola SK (2015) Analysis of skin cancer in albinos Ibadan ; Nigerian J Plast Surg 11: 23–28.
- Traoré B, Keita M, Condé M (2016) Skin cancers clinicopathological features in the surgical oncology unit of Conakry Teaching Hospital. Rev CMES santé 2: 2424–7243.
- Fritz A, Percy C, Jack A et al. (2000) International Classification of Diseases for oncology. World Health Organization Geneva. Third Edition 2000
- Sobin LH, Gospodarowier MK, Wittekind CH (2010) Classification des tumeurs malignes UICC CASSINI 7e édition 173–174 ; 180.
- Kromberg JG, Castle D, Zwane EM et al. Albinism and skin cancer in Southern Africa. Clin Genet 1989 36: 43–52.
- Opara KO, Jibururum BC (2010) Skin cancers in albinos in a teaching Hospital in eastern Nigeria – presentation and challenges of care; Word J Surg Oncol 8: 73.
- Eisenhauer EA, Therasse P, Bogaerts J (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version1.1). Eur J Cancer 45: 228–247.
- Dusingize JC, Olsen CM, Pendeya NP (2017) Cigarette smoking and the Risks of Basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 137: 1700–1708.
- Emadi SE, Suleh AJ, Babamamoodi F (2017) Common malignant cutaneous among albinos in Kenya. Med J Islam Republ Iran 31.1
- Lekalakala PT, khammissa RAG, Kramer B (2015) Oculocutaneous Albinism and Squamous Cell Carcinoma of the Skin of the Head and Neck in Sub-Saharan Africa,” Journal of Skin Cancer 2015 : 6.
- Onuigbo W (2015) The Recognition of recurrent from of albino skin cancer. J cancer Prev Cur Res 3(1)
- Asuquo ME, Otei OO, Omotoso J, Bassey EE (2010) Letter: Skin cancer in albinos at the University of Calabar Teaching Hospital, Calabar, Nigeria. Dermatol Online J 16: 14. [crossref]
- Graziosi GB, Sbachiero JC, Neto BRC (2014 ) Diagnostique et traitement pour le cancer de la peau chez les albinos : une etude Descriptive. Brazilian journal of plastic surgery 26:1983–5175
- Ofilli O (2014) Recurrence of Rhadomyo sarcoma in squamous cell carcinoma in Nigeran pal skinned person (albinos) : A case report. Advanced Research Journal of Medical Sciences 1: 058–060.
- Traore B et Lamah L (2017) Outcomes of surgical treatment of skin cancer at surgical oncology of Donka Conakry University hospital. Journal of cancer therapy 8 : 1086–1094.
- Hong ES, Zeeb H, Repacholi MH (2006) Albinism in Africa as a public health issue. BMC Public Health 6: 212. [crossref]
- Berger E, Hunt R, Tzu J (2011) Squamous-cell carcinoma in situ in a patient with oculocutaneous albinism. Dermatology Online 17: 22.
- Chidothe IA, Masamba L. Neoadjuvant chemotherapy in Albinos with advanced skin cancer at a Blantyre Hospital: Case series. Malawi Medical Journal 23 : 97–99.
- Mapurisa G1, Masamba L (2010) Locally advanced skin cancer in an albino: a treatment dilemma. Malawi Med J 22: 122–123. [crossref]