DOI: 10.31038/CST.2017246
Commentary
The “biogenea pharmaceuticals” being the first biotech company in Southeast Europe, has the task to systematically promote the Science of Pharmaceutical Biotechnology, including the production of advanced therapy medicinal products ATMPs as defined by the European Medicines/Agency EMEA. On behalf of “biogenea pharmaceuticals” and its scientific board we are pleased to announce our innovative biotechnological services Nanostemogenea™ for the collection, processing, cryopreservation and therapeutic use of our advanced biotechnology applications utilizing next generation biocompatible complexes of stem, immune cells and nanoparticles in the field of magnetic guided regenerative medicine. Our GENEA cells ™ are totally safe and obtained from different sources of the human body for autologous cell therapy purposes in Renaissance medicine.
Biogenea Pharmaceuticals™ is the first Inter-Balkan Pharmaceutical Biotechnology Company since leading the way since 2005 in Red Biotechnology applications, in Cryobiology and in Autologous Cellular Therapy of Degenerative Diseases (cardiological diseases, neurological conditions and metabolic disorders).
Biogenea Pharmaceuticals focuses
- On the collection, processing, cryopreservation and cGMP (according to Good Manufacturing Practice) production -for solely autologous use – of cellular therapeutical solutions from blood (bone marrow, peripheral blood, cord blood) or blood compounds for human use.
- In collaboration with Regenetech on stem cell expansion technologies, which were created in the research laboratories of NASA (National Aeronautics and Space Administration).
- On the cGMP production of advanced medicinal products (1394/2007/ΕC) for solely autologous use from skin, dental pulp, cord tissue). (In preclinical-research phase: 2008-2009).
- On certified genetic analyses in collaboration with International Referral Centers.
- On copyright protection according to the American and/or European Copyright Agency. The “biogeneapharmaceuticals” is the only European bank that has been recognized by the European Medicines Agency EMEA as a pharmaceutical company and has the possibility of cryopreservation of hematopoietic stem cells and conducting clinical trials (EMEA/Qualification of an enterprise as an SME – GrigoriadisBros – Biogenea- Cellgenea Ltd, with registration number: EMA/SME/084/10).
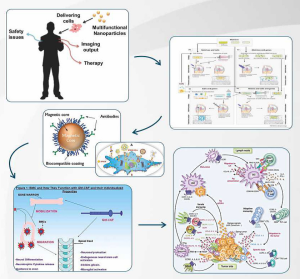
In “biogenea pharmaceuticals” taking advantage of the unique properties of super paramagnetic nanoparticles as this high magnetic moment and susceptibility but also the existence yperparagnitikis behavioural development multitude biological applications agglomerate formation with these petidika molecular and different kinds STEM CELL for autologous use in the Renaissance medicine.
Biogenea Pharmaceuticals Ltd combines excellent trained scientific personnel with the most modern techniques, concerning cell expansion, that are used today in the field of biotechnology and have been developed by NASA. Thus our company is able to verge into the demanding field of clinical trials concerning stem cell treatments. One can understand the big potential of these stem cells to play a role in Regenerative Medicine by looking at the amount of clinical trials all over the world that use stem cells as a way to treat an increasing number of diseases in a supportive manner.
The “biogenea pharmaceuticals” provides the following services:
- Cardiogenea™: Autologous intracoronary, intraarterial or intracardiac injection of autologous blood, bone marrow and heart stem cells derive active cardiopoeitic spheres coated with superparamagneticnanoparticles for the restoration of myocardial infarction.
- Dendrigenea™: Autologous adjuvant immune hybridomatic therapy for cancer patients using advanced complexes of superparamagnetic iron oxide nanoparticles coated mature dendritic and dendritic tumor cell fusions as a cancer ‘cell vaccine’.
- Cartigenea™: Autologous and exvivo expanded chondrogenic cells coated with super paramagnetic nanoparticles for their intended use in Cartilage Defects.
Biogenea Pharmaceuticals Ltd is in the process of developing novel therapies for the effective treatment of several diseases. These therapies make use of Stem Cells (ADSCs) and a special class of nanoparticles, referred to as Super paramagnetic Iron Oxide Nanoparticles (SPIONs). SPIONs have recently attracted the interest of the scientific community, due to several attributes, such as their paramagnetism and their potential us in a number of therapeutic approaches. SPIONs are small synthetic γ-Fe2O3 (maghemite), Fe3O4 (magnetite) or α-Fe2O3 (hermatite) particles with a core ranging from 10 nm to 100 nm in diameter. In addition, mixed oxides of iron with transition metal ions such as copper, cobalt, nickel, and manganese, are known to exhibit superparamagneticproperties and also fall into the category of SPIONs. However, magnetite and maghemite nanoparticles are the most widely used SPIONs in various biomedical applications. SPIONs have an organic or inorganic coating so that they can be tolerated by cells and tissues.
Our Personalized cancer therapy is determined by the needs and specificities of a particular oncology patient to provide the optimum desired therapeutic effect with minimal toxicity, strengthening the immune system of cancer patients with the use TARGETED complex dendritic cells and super-paramagnetic nanoparticles in simultaneous increase the concentration of magnetic particles into the tumor.
Our technique serves the philosophy of personalized cancer treatment based on tumor pluripotent cells (cancer stem cells), consisting of four specialized areas in the prevention, diagnosis, treatment and rehabilitation of cancer patients while capitalizing on the therapeutic effects of magnetic hyperthermia the implementation of magnetic field for the local heating of the cancer cells with a view to the disaster.
Preventive Cryopreservation Insurance of Primordial Cells & Therapeutic Applications
- Continuous update and prompt training of interested Donors and Clinicians of Public and Private Health Centers and Hospitals, about the progenitor cells applications.
- Immediate availability of the stored samples 365 days a year, 7 days a week, 24 hours a day.
- Complete attunement with the specifications set by the European Accreditation Organization for cellular therapy (FACT/JACIE/ NETCORD), the American Association of Blood Banks (AABB) and the relevant Greek.
- Presidential Decretal 2004/23 for the tissue/cell banks, with the use of cGMP methods.
- Control of Plasticity of progenitor cells based on the detailed Validation Master Plan of the Standard Operating Procedures (SOPs) regarding their hematopoietic origin (Methocult).
- Fully Automated Viability Control of the cryopreserved progenitor cells with automatic luminometric device, which increases the reproducibility and the accuracy of the results in contrast to the common laboratory techniques for detecting dead cells microscopically (Trypan Blue staining).
- Validated Molecular Diagnostics service provision (detection of HIV1/2, HBV, HCV, CMV, Syphillis, Toxoplasma) applying Real- Time PCR technology (Roche LightCycler).
- Validated ex-vivo cellular and/or tissue expansion of the recently processed cells/tissues, as well as of the cryopreserved cell/ tissues in advanced technology Bioreactors, created in the NASA research laboratories, offering unique micro-gravity conditions in alternating electromagnetic field! Cellgenea is the UNIQUE company in Greece able to expand and to use ex-vivo expanded cord blood and adult progenitor cells for therapeutic reasons and clinical trials thanks to the relevant know-how transfer from the research laboratories of NASA and Regenetech Biotechnology Company.
- Storing of progenitor cells in two-chambered bags and cryovials in complete (24 hours a day) controlled and automated liquid nitrogen tanks.
- Continuous control and cross-tracking of the cryopreserved samples with validated LIS-ERP software which ensures the ability to track down and to identify the sample during all the steps of its supply, the processing, the control, the storage and the distribution. The tracking is also used for controlling and identifying all the relevant data about the products and the materials that come in contact with these samples (2004/23/ΕΚ).
- Immediate availability and distribution of the sample inside a validated cryotank, in case of therapeutic application.
- Strict security concerning personal data, confidentiality and safety according to the Personal Data Protection Agency.
- Complete bacteriological and serological control of the samples using the automatic analyzers BacT/ALERT and Architect i1000 without any extra rate.
- Life insurance provided to all the members of the different programs of preventive cryopreservation (Cellgenea, Dentogenea, Dermigenea, Angiogenea, DΝΑgenea, Neurogenea, Cardiogenea) in cooperation with insurance companies.
- DNA & pharmacogenetic control services for personalized treatment without side effects.
- Offer of validated prenatal and DNA/RNA control for chromosomal anomalies screening in a variety of biological materials (adult peripheral blood, cord blood, tissue biopsies) in cooperation with the Technological Park of Ioannina.
- Continuous training and schooling of the scientific staff on the new
Nanuristemogenea™
Advanced biotechnology applications utilizing next generation biocompatible complexes of stem and hybridoma immune cells with nanoparticles in the field of magnetic guided regenerative medicine.
The “biogenea pharmaceuticals” being the first biotech company in Southeast Europe, has the task to systematically promote the Science of Pharmaceutical Biotechnology, including the production of advanced therapy medicinal products ATMPs as defined by the European Medicines/Agency EMEA.
On behalf of “biogenea pharmaceuticals” and its scientific board we are pleased to annpounce our innovative biotechnological services Nanostemogenea™ for the collection, processing, cryopreservation and therapeutic use of our advanced biotechnology applications utilizing next generation biocompatible complexes of stem, immune cells and nanoparticles in the field of magnetic guided regenerative medicine. Our GENEAcells™ are totally safe and obtained from different sources of the human body for autologous cell therapy purposes in Renaissance medicine.
Biogenea Pharmaceuticals™ is the first Inter-Balkan Pharmaceutical Biotechnology Company since leading the way since 2005 in Red Biotechnology applications, in Cryobiology and in Autologous Cellular Therapy of Degenerative Diseases (cardiological diseases, neurological conditions and metabolic disorders).
Biogenea Pharmaceuticals focuses
- On the collection, processing, cryopreservation and cGMP (according to Good Manufacturing Practice) production -for solely autologous use – of cellular therapeutical solutions from blood (bone marrow, peripheral blood, cord blood) or blood compounds for human use.
- In collaboration with Regenetech on stem cell expansion technologies, which were created in the research laboratories of NASA (National Aeronautics and Space Administration).
- On the cGMP production of advanced medicinal products (1394/2007/ΕC) for solely autologous use from skin, dental pulp, cord tissue). (In preclinical-research phase: 2008-2009).
- On certified genetic analyses in collaboration with International Referral Centers.
- On copyright protection according to the American and/or European Copyright Agency. The “biogeneapharmaceuticals” is the only European bank that has been recognized by the European Medicines Agency EMEA as a pharmaceutical company and has the possibility of cryopreservation of hematopoietic stem cells and conducting clinical trials (EMEA/Qualification of an enterprise as an SME – GrigoriadisBros – Biogenea- Cellgenea Ltd, with registration number: EMA/SME/084/10).
- In “biogenea pharmaceuticals” taking advantage of the unique properties of super paramagnetic nanoparticles as this high magnetic moment and susceptibility but also the existence yperparagnitikis behavioral development multitude biological applications agglomerate formation with these petidika molecular and different kinds STEM CELL for autologous use in the Renaissance medicine.
- Biogenea Pharmaceuticals Ltd combines excellent trained scientific personnel with the most modern techniques, concerning cell expansion, that are used today in the field of biotechnology and have been developed by NASA. Thus our company is able to verge into the demanding field of clinical trials concerning stem cell treatments. One can understand the big potential of these stem cells to play a role in Regenerative Medicine by looking at the amount of clinical trials all over the world that use stem cells as a way to treat an increasing number of diseases in a supportive manner.
- The “biogenea pharmaceuticals” provides the following services:
- Cardiogenea™: Autologous intracoronary, intra-arterial or intracardiac injection of autologous blood, bone marrow and heart stem cells derive active cardiopoeitic spheres coated with super paramagnetic nanoparticles for the restoration of myocardial infarction.
- Dendrigenea™: Autologous adjuvant immune hybridomatic therapy for cancer patients using advanced complexes of super paramagnetic iron oxide nanoparticles coated mature dendritic and dendritic tumor cell fusions as a cancer ‘cell vaccine’.
- Cartigenea™: Autologous and ex-vivo expanded chondrogenic cells coated with super paramagnetic nanoparticles for their intended use in Cartilage Defects.
Biogenea Pharmaceuticals Ltd is in the process of developing novel therapies for the effective treatment of several diseases. These therapies make use of Stem Cells (ADSCs) and a special class of nanoparticles, referred to as Super paramagnetic Iron Oxide Nanoparticles (SPIONs). SPIONs have recently attracted the interest of the scientific community, due to several attributes, such as their paramagnetism and their potential us in a number of therapeutic approaches. SPIONs are small synthetic γ-Fe2O3 (maghemite), Fe3O4 (magnetite) or α-Fe2O3 (hermatite) particles with a core ranging from 10 nm to 100 nm in diameter. In addition, mixed oxides of iron with transition metal ions such as copper, cobalt, nickel, and manganese, are known to exhibit super paramagnetic properties and also fall into the category of SPIONs. However, magnetite and maghemite nanoparticles are the most widely used SPIONs in various biomedical applications. SPIONs have an organic or inorganic coating so that they can be tolerated by cells and tissues.
Our Personalized cancer therapy is determined by the needs and specificities of a particular oncology patient to provide the optimum desired therapeutic effect with minimal toxicity, strengthening the immune system of cancer patients with the use TARGETED complex dendritic cells and super-paramagnetic nanoparticles in simultaneous increase the concentration of magnetic particles into the tumor.
Our technique serves the philosophy of personalized cancer treatment based on tumor pluripotent cells (cancer stem cells), consisting of four specialized areas in the prevention, diagnosis, treatment and rehabilitation of cancer patients while capitalizing on the therapeutic effects of magnetic hyperthermia the implementation of magnetic field for the local heating of the cancer cells with a view to the disaster.
Preventive Cryopreservation Insurance of Primordial Cells & Therapeutic Applications
- Continuous update and prompt training of interested Donors and Clinicians of Public and Private Health Centers and Hospitals, about the progenitor cells applications.
- Immediate availability of the stored samples 365 days a year, 7 days a week, 24 hours a day.
- Complete attunement with the specifications set by the European Accreditation Organization for cellular therapy (FACT/JACIE/ NETCORD), the American Association of Blood Banks (AABB) and the relevant Greek Presidential Decretal 2004/23 for the tissue/cell banks, with the use of cGMP methods.
- Control of Plasticity of progenitor cells based on the detailed Validation Master Plan of the Standard Operating Procedures (SOPs) regarding their hematopoietic origin (Methocult).
- Fully Automated Viability Control of the cryopreserved progenitor cells with automatic luminometric device, which increases the reproducibility and the accuracy of the results in contrast to the common laboratory techniques for detecting dead cells microscopically (Trypan Blue staining).
- Validated Molecular Diagnostics service provision (detection of HIV1/2, HBV, HCV, CMV, Syphillis, Toxoplasma) applying Real- Time PCR technology (Roche Light Cycler).
- Validated ex-vivo cellular and/or tissue expansion of the recently processed cells/tissues, as well as of the cryopreserved cell/tissues in advanced technology Bioreactors, created in the NASA research laboratories, offering unique micro-gravity conditions in alternating electromagnetic field! Cellgenea is the UNIQUE company in Greece able to expand and to use ex-vivo expanded cord blood and adult progenitor cells for therapeutic reasons and clinical trials thanks to the relevant know-how transfer from the research laboratories of NASA and Regenetech Biotechnology Company.
- Storing of progenitor cells in two-chambered bags and cryovials in complete (24 hours a day) controlled and automated liquid nitrogen tanks.
- Continuous control and cross-tracking of the cryopreserved samples with validated LIS-ERP software which ensures the ability to track down and to identify the sample during all the steps of its supply, the processing, the control, the storage and the distribution. The tracking is also used for controlling and identifying all the relevant data about the products and the materials that come in contact with these samples (2004/23/ΕΚ).
- Immediate availability and distribution of the sample inside a validated cryotank, in case of therapeutic application.
- Strict security concerning personal data, confidentiality and safety according to the Personal Data Protection Agency.
- Complete bacteriological and serological control of the samples using the automatic analyzers BacT/ALERT and Architect i1000 without any extra rate.
- Life insurance provided to all the members of the different programs of preventive cryopreservation (Cellgenea, Dentogenea, Dermigenea, Angiogenea, DΝΑgenea, Neurogenea, Cardiogenea) in cooperation with insurance companies.
- DNA & pharmacogenetic control services for personalized treatment without side effects.
- Offer of validated prenatal and DNA/RNA control for chromosomal anomalies screening in a variety of biological materials (adult peripheral blood, cord blood, tissue biopsies) in cooperation with the Technological Park of Ioannina.
- Continuous training and schooling of the scientific staff on the new scientific developments.